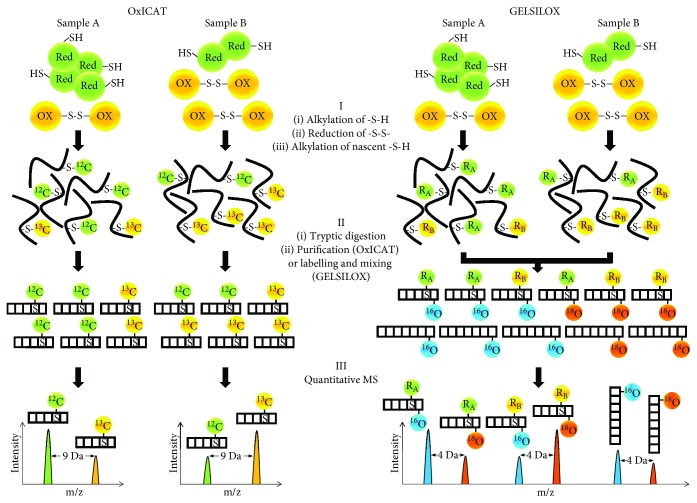
Figure 3.
The OxICAT and GELSILOX approaches to redox proteomics. Step I: a hypothetical protein, which exists in two different samples A and B in both the reduced (green) and oxidized (disulfide-linked, yellow) form, is incubated with an alkylating agent (12C-IAM for OxICAT, left, and, e.g., IAM, for GELSILOX, right) to block free sulfhydryl groups. Then, a reducing agent is used to cleave the disulfide bonds and the nascent thiol groups blocked using a second alkylating agent (13C-IAM for OxICAT and, e.g., NEM, for GELSILOX). Step II: the proteins are digested with trypsin and the so-obtained peptides either affinity-purified (OxICAT) or tagged with 18O labelling prior to mixing (GELSILOX). Step III: two independent quantitative MS assays reveal the extent of thiol modification in samples A and B (OxICAT), whereas one single MS assay yields not only the relative amount of the reduced and oxidized forms but also the relative quantitation of the corresponding protein between the two samples based on the MS signals from noncysteine peptides.