Fig. 1.
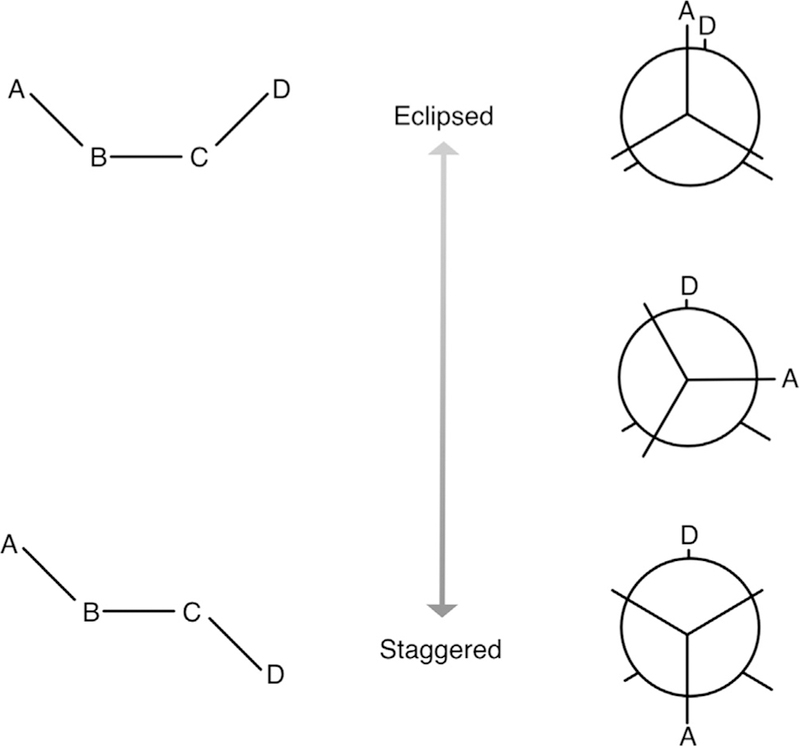
Notional diagram of butane. The four carbon atoms are labeled A, B, C, and D, and hydrogens are omitted for clarity. Again ignoring hydrogens, bond terms are A–B and C–D, which would be equivalent, and B–C. Angle terms are A–B–C and B–C–D, also equivalent, and the dihedral term is A–B–C–D. The eclipsed version of the dihedral is at the top and staggered at the bottom. Newman projections are at the right. The eclipsed conformation is higher energy than the staggered conformation, because carbons A and D are further apart in the staggered conformation.
