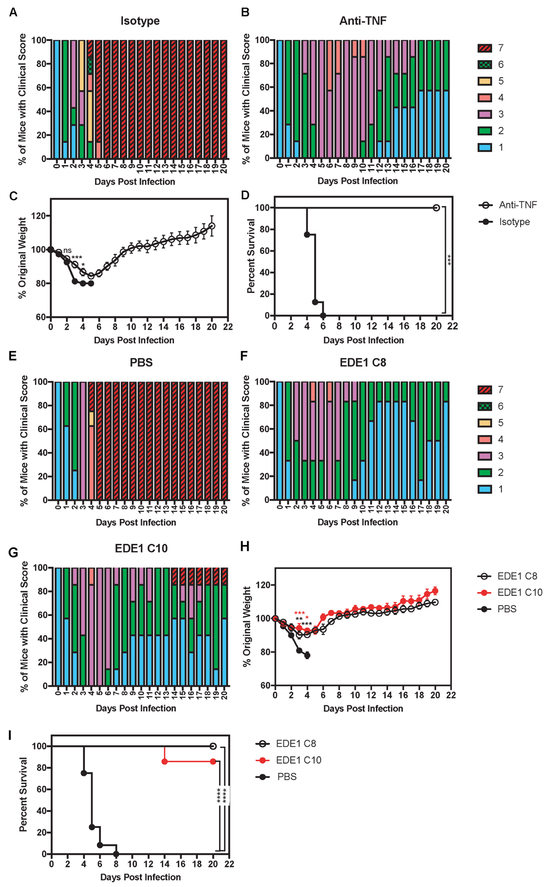
Figure 4. Administration of anti-TNF Ab or EDE1 C8 and C10 Abs, which recognize EDE1 epitopes, prevents lethal dengue disease in mice born to ZIKV-immune mothers.
Four- to 5-week-old LysMCre+Ifnar1fl/fl mice born to mothers infected with ZIKV strain SD001 (1×106 FFU via retro-orbital route) for 7-9 months (n=3) were treated via an intraperitoneal injection with 100 μg of isotype control Ab (clone HPRN) or anti-TNF Ab (clone XT3.11) on days 1, 2, and 3 following inoculation with 106 FFU of DENV2 strain S221 via tail vein injection. (A) Clinical scores of isotype control Ab-treated mice (n=7), (B) Clinical scores of anti-TNF Ab-treated mice (n=8), and (C) Weight loss and (D) Survival rates of isotype control Ab-treated mice (black circles) and anti-TNF Ab-treated mice (open circles). Administration of EDE1 C8 or EDE1 C10 Abs was performed in 4- to 5-week-old LysMCre+Ifnar1fl/fl mice born to mothers infected with ZIKV strain SD001 (1×106 FFU via retro-orbital route) for 12-13 months (n=4). These pups were injected via an intraperitoneal route with PBS, EDE1 C8 Ab (100 μg), or EDE1 C10 Ab (100 μg) on days 1, 2, and 3 following challenge with DENV2 strain S221 (106 FFU via tail vein). (E) Clinical scores of PBS control mice (n=9), (F) Clinical scores of EDE1 C8 Ab-administered mice (n=10), (G) Clinical scores of EDE1 C10 Ab-administered mice (n=8), and (H) Weight loss and (I) Survival rates of PBS-treated (black circles), EDE1 C8-treated (open circles), and EDE1 C10-treated (red circles) mice. Data are pooled from two independent experiments and are expressed as mean ± standard error of mean. (C and H) Unpaired Student’s t test of groups for each day. (D and I) log-rank test * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.