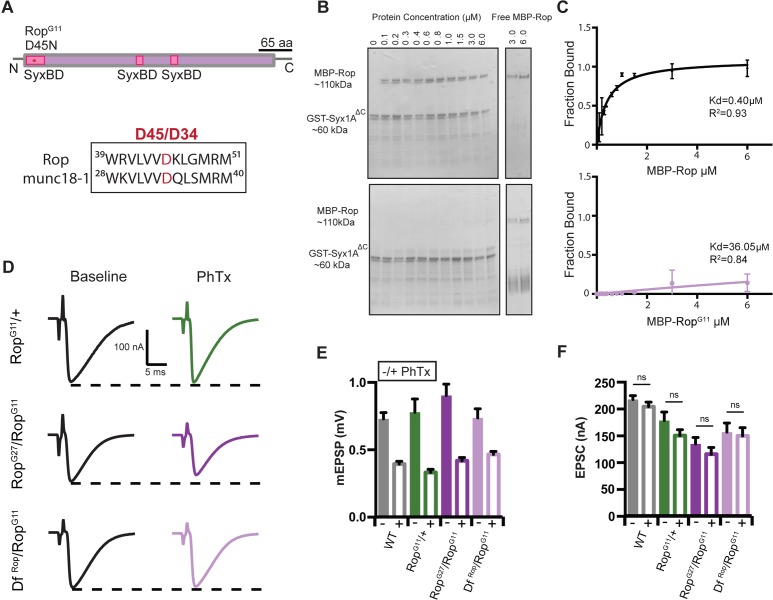
Figure 9. RopG11 abolishes the biochemical interaction between Rop and syx1A and rescues homeostasis defect in Rop.
(A) Schematic of the Drosophila Rop protein. Point mutation RopG11 is indicated by red star at syntaxin-binding domain (SBD) of the Rop protein shown in pink. RopG11 converts Aspartic Acid (D45) to Asparagine (N). This site is conserved in mammalian Rop (munc18-1). (B) Coomassie stains of in vitro binding assays. (left) MBP-Rop (110 kDa) coprecipitated with bead-bound GST fusions of syx1A (GST-syx1AΔC) (60 kDa). MBP-Rop does not bind to GST-syx1AΔC in the presence of single point mutation at the N-terminal of Rop (MBP-RopG11). (right) Free MBP-Rop in the absence of GST-syx1A. (C) Binding curves quantify dissociation constant (Kd) for MBP-Rop and MBP-RopG11 binding to GST-syx1AΔC; x-axis is concentration of MBP recombinant protein used (μM); y-axis is the fraction of protein bound; n = 2 (D) Sample traces showing EPSC amplitudes ± PhTx for RopG11 heterozygous null allele (RopG11/+), heterozygous RopG11 placed in trans with RopG27 mutant (RopG27/RopGG11), and heterozygous RopG11 placed in trans with DfRop (DfRop/RopGG11). (E) Average data for mEPSP amplitude ±PhTx for indicated genotypes. PhTx application reduces amplitude in all genotypes (p<0.01). Data represent mean ±SEM. Student’s t test. two tailed. (F) Average data for EPSC amplitude as in (B); ns, not significant; Student’s t test, two tailed.