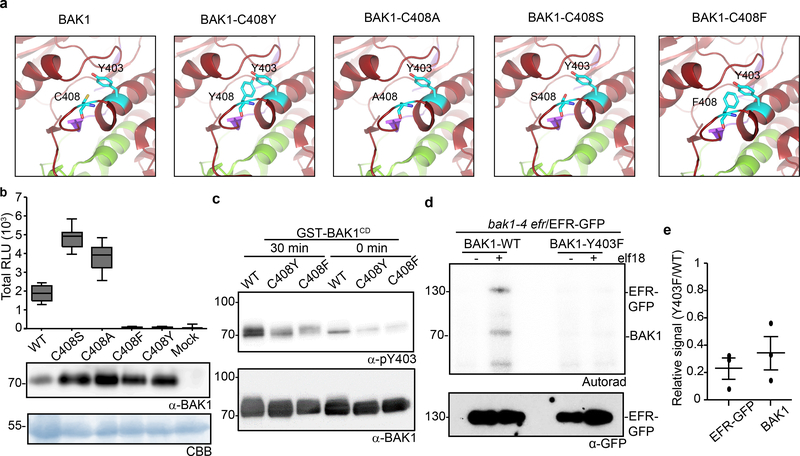
Figure 3 |. Phosphorylation of the conserved BAK1-Tyr403 residue is important for ligand-induced activation of immune receptor complex.
a, In silico substitutions of BAK1-C408 on a selected region of the BAK1 cytoplasmic domain (BAK1CD) structure (3UIM.pdb). The activation segment (green) and catalytic loop (purple) are highlighted. b, Total ROS production following treatment with 100 nM flg22 over 60 min of n=12 biological independent suspensions of bak1–4 mesophyll protoplasts transiently expressing the indicated BAK1 mutants. Measurements are plotted as boxplots displaying the first and third quartiles, split by the median; whiskers extend to a maximum of 1.5 × interquartile range beyond the box. c, In vitro Y403 phosphorylation of recombinant BAK1CD proteins after a 30-minute kinase reaction as detected by α-pY403. Blots were further probed with α-BAK1 for loading control. b, c, Experiments were repeated independently three times. d, Phosphorylation of EFR-GFP and associated BAK1 after treatment with water (−) or 100 nM elf18 (+) for 10 min. EFR-GFP was immuno-precipitated using GFP-Trap beads and then submitted to an in vitro kinase assay with [32P]γ-ATP. EFR-GFP levels were determined by western blot analysis with α-GFP antibodies. b-d, For gel source data, see Supplementary Figure 1. e, Aligned dot blots show the relative reductions in BAK1 and EFR-GFP phosphorylation levels in the bak1–4/Y403F/EFR-GFP sample, as measured in three independent experiments of in vitro kinase assays as in (d). Line represent mean and error bar SE.