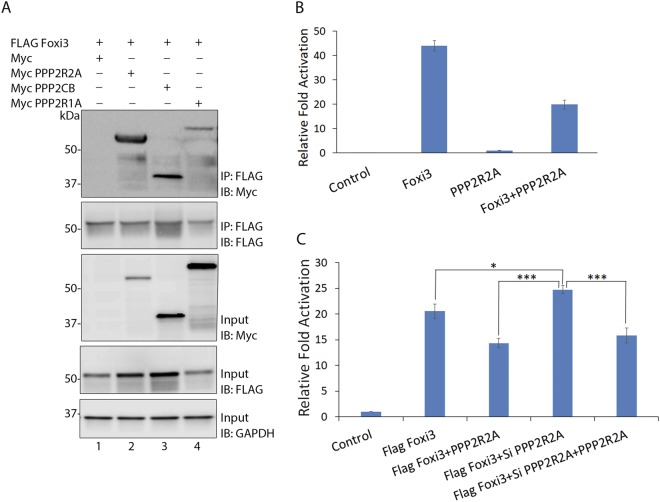
Figure 6.
Foxi3 is negatively regulated by its interaction with the PP2A complex. (A) Co-IP showing the interaction between Foxi3 and various PP2A subunits. Myc-tagged PP2A subunits, PPP2R2A, PPP2CB and PPP2R1A were transfected along with FLAG Foxi3. Anti-Flag M2 magnetic beads were used to perform immunoprecipitations and MYC was used to detect the PP2A subunits. Inputs are shown to validate the expression of indicated constructs and GAPDH is used to show equal amounts of lysate were used for each immunoprecipitation. Full-length blots are presented in Supplementary Fig. 2. (B) Reporter assay showing that co-expression of PPP2R2A reduces the Foxi3-mediated activation of the AE4 luciferase reporter. PPP2R2A itself does not have any effect on the reporter activity. (C) Expression of exogenous PPP2R2A attenuates Foxi3 reporter activation. In contrast, Foxi3-mediated reporter activation is increased by knockdown of endogenous PPP2R2A in HEK-293T cells. The effect of siRNA knockdown of PPP2R2A on Foxi3 is reversed by overexpression of a PPP2R2A cDNA. Each experiment was performed in triplicate and was repeated at least three times. P-values are calculated Results are expressed as the mean ± SD calculated from triplicates. Statistical significance was determined by Student’s t test. A value of P < 0.05 was considered as statistically significant (*P < 0.05; ***P < 0.0005). IB: Immunoblotting; IP: Immunoprecipitation; kDa: kiloDalton.