Abstract
Polycystic ovary syndrome (PCOS) presents with a spectrum of conditions resulting from androgen excess, anovulation and metabolic syndrome. Patients with PCOS may see their primary care physicians for various presentations, including hirsutism, acne, menstrual irregularities, infertility, obesity, and psychiatric disorders such as anxiety and depression. Management of these patients should include screening for Type 2 diabetes mellitus, dyslipidaemia and hypertension. Treatment should be targeted to each patient’s phenotype and personal expectations such as desire for pregnancy. Psychological well-being due to the effects on physical appearance is also an important consideration. Diet and exercise are major components in the management of patients with PCOS and obesity. The first-line therapy for fertility and metabolic syndrome in PCOS is lifestyle modification with diet and exercise, followed by pharmacological therapy.
Keywords: anovulation, hirsutism, hyperandrogenism, infertility, polycystic ovary syndrome
You were surprised when Andrea, an 18-year-old student, visited your clinic without her mother for the first time. She said she wanted to seek your advice for her frequent bad acne outbreaks. She also asked if there were any medications that could make the hair on her hands appear less obvious. You astutely asked her if her menses had been irregular and sensitively discussed why her acne and hirsutism were bothering her.
WHAT IS POLYCYSTIC OVARY SYNDROME?
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting young women. It can present with a wide spectrum of signs and symptoms, including acne, hirsutism, obesity, menstrual irregularities and infertility. These are caused by androgen excess, anovulation, insulin resistance and the ensuing metabolic syndrome. The precise aetiology of PCOS is still unclear but is likely to be a complex interaction of genetic and environmental factors. A family history of PCOS confers a higher risk of developing the condition. Other contributing factors include low birth weight, premature pubarche, obesity, diabetes mellitus (DM) and antiepileptic drug use.(1)
Due to its many manifestations throughout the course of a woman’s life, PCOS can have a great impact on the individual’s metabolic, cardiovascular, reproductive and psychological well-being. Hence, it is an important syndrome to recognise and treat appropriately.
HOW RELEVANT IS THIS TO MY PRACTICE?
PCOS has an estimated prevalence of 6%–10%.(2) Patients typically present to primary care with acne and menstrual irregularities. Anxiety and depression are other more insidious symptoms of PCOS that are important to recognise early. Additionally, PCOS is one of the most common causes of anovulation, so patients may first present with difficulty conceiving. As PCOS is associated with insulin resistance, all women with PCOS should be screened for DM or pre-DM, especially if they are planning to conceive, as poorly controlled DM is associated with adverse pregnancy outcomes.
WHAT CAN I DO IN MY PRACTICE?
Detection
It is important to consider PCOS in any woman of reproductive age who presents with symptoms of androgen excess (e.g. acne, hirsutism) and/or menstrual irregularities. While obesity is associated with this condition, it should be noted that the majority of patients with PCOS in Singapore have normal body weight.(3)
Diagnosis
Contrary to popular belief, the presence of polycystic ovaries on ultrasonography is not essential in the diagnosis of PCOS. Different diagnostic criteria have been used to define PCOS since 1990, among which the 2003 Rotterdam criteria(4) are currently recommended. Based on these criteria, a diagnosis of PCOS requires two out of three of the following: hyperandrogenism, menstrual irregularities and polycystic ovaries on ultrasonography (Fig. 1).
Fig. 1.
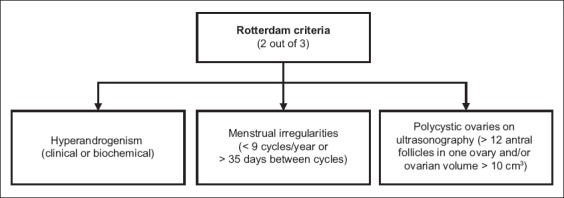
Flowchart shows the Rotterdam criteria for diagnosis of polycystic ovary syndrome.(4)
Hyperandrogenism can be defined either clinically or biochemically. Clinical features include hirsutism, acne and male pattern hair loss. Hirsutism may be graded based on the Ferriman-Gallwey score, but it is important to bear in mind normal ethnic differences in hair distribution; while a score of ≥ 8 is taken to be abnormal in Caucasian women, a score of ≥ 5 may be a more appropriate cut-off for those of Chinese ethnicity.(5) Biochemical hyperandrogenism is defined as elevated serum total testosterone or calculated free testosterone levels. Currently available direct assays for free testosterone are of limited value. Menstrual irregularities can be oligomenorrhoea (cycles of > 35 days but < 6 months apart) or amenorrhoea (absence of menstruation for 6–12 months after a cyclic pattern has been established). Polycystic ovary morphology on ultrasonography is defined as an ovary containing 12 or more follicles measuring 2–9 mm in diameter and/or an ovary with a volume greater than 10 mL.
Diagnosis of PCOS requires the exclusion of other disorders such as pregnancy, thyroid dysfunction, hyperprolactinaemia, Cushing’s syndrome, non-classical congenital adrenal hyperplasia and androgen-secreting tumours. In women with symptoms or signs of androgen excess, serum total testosterone should be checked and these women referred to a specialist for evaluation if the level is over two times the upper limit of normal. Depending on the clinical picture, further tests may be necessary (Box 1). These may include beta-HCG (beta-human chorionic gonadotropin), thyroid function, prolactin, 1 mg overnight dexamethasone suppression and early morning serum 17-OHP (17-hydroxyprogesterone) tests.(6) Mild elevations in serum prolactin are common in PCOS, but after excluding macroprolactin, levels that are greater than twice the upper limit of normal should warrant further investigation. High levels of anti-Müllerian hormone, a hormone produced by ovarian follicle granulosa cells, are also seen in PCOS and may be useful in the diagnosis of the condition.(7)
Box 1.
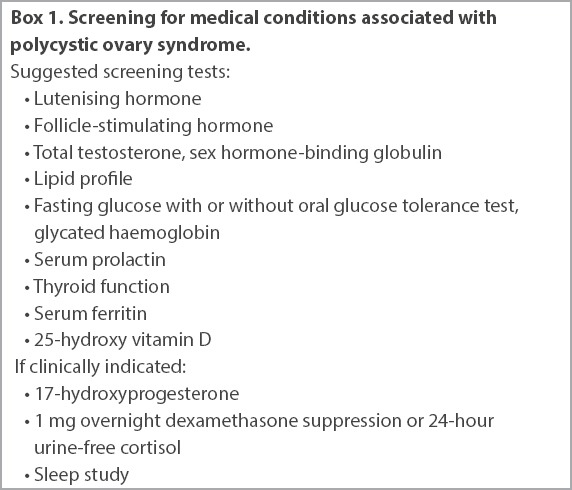
Screening for medical conditions associated with polycystic ovary syndrome.
Screening
Women with PCOS are at high risk for developing Type 2 diabetes mellitus (T2DM) and insulin resistance. Routine screening for T2DM, impaired fasting glucose and impaired glucose tolerance should be carried out using a standard oral glucose tolerance test. Fasting lipid profile should also be measured, while blood pressure should be taken at each visit.
Screening for coronary artery disease and obstructive sleep apnoea (OSA) can be considered in women who are at high risk. Obesity increases the risk of endometrial cancer by up to threefold in women with PCOS.(8) Routine screening for endometrial cancer using ultrasonography is not currently recommended.(6) However, it is important to have a high index of suspicion for patients with prolonged oligomenorrhoea (> 3 months between menses).
Iron deficiency is common, and may contribute to fatigue and androgenic alopecia, so we suggest screening and treatment with iron, targeting serum ferritin in the upper quartile of the reference range.(9) Vitamin D deficiency is common in patients with PCOS, and this may have an additive adverse effect on fertility, insulin resistance and glucose intolerance,(10) and thus screening for and replacing any deficiency may be helpful. Psychological well-being due to the effects of PCOS on physical appearance (e.g. weight gain, acne and hirsutism) is also an important consideration. Attention should be given to actively looking for mental health issues such as depression, anxiety and self-harm.
Self-management
The first-line therapy for women with PCOS and obesity is lifestyle modification in the form of diet and exercise. Strong associations exist among excessive weight, insulin resistance, glucose intolerance, menstrual irregularities and infertility. Even modest lifestyle changes can have a significant impact, and reducing body weight by only 2%–5% has been shown to restore ovulation and increase insulin sensitivity in obese anovulatory women.(11) Weight reduction has additional benefits and reduces the risk of DM, hypertension, cardiovascular disease, OSA and certain malignancies.
Pharmacological management
Aside from lifestyle modifications, treatment for PCOS has to be multi-targeted to suit each patient’s phenotype, symptoms, personal goals and expectations, such as desire for pregnancy. Pharmacological management is discussed herein according to symptoms. If there are no immediate pregnancy plans, management is dependent on the symptoms present.
Menstrual irregularities
Patients with oligomenorrhoea have an increased risk of endometrial hyperplasia, and pharmacological therapy may be necessary to induce a withdrawal bleed if the interval between menses is longer than two months. To induce periods, cyclical progesterone can be used, such as Duphaston (dydrogesterone 10 mg twice daily for a week) every two months to ensure regular shedding of the endometrium. The oral contraceptive pill (OCP) is also effective in controlling menses, with the added benefit of providing contraception and improving androgenic symptoms. However, many women with PCOS are obese and this, together with the OCP, confers an increased risk of thrombosis, so patient selection is important. It is advisable to avoid OCPs with higher oestrogen doses or those containing 19-norprogesterone derivatives, as these androgenic progestins may adversely affect the patient’s cardiovascular risk.(9) Treatment needs to be individualised, but we suggest starting with low-dose ethinylestradiol combined with a third-generation progestin (i.e. desogestrel, gestodene or norgestimate) or fourth-generation progestin (i.e drospirenone), as these have the least intrinsic androgenic activity. Metformin can help to restore menstrual cyclicity and may be given concurrently with the above.
Androgen excess
Hyperandrogenism in PCOS is driven by insulin resistance, hypersecretion of luteinising hormone (LH) and ovarian androgens. Medications commonly used to treat androgen excess target these pathways. For example, spironolactone is an anti-androgen that blocks the effect of testosterone at the level of the androgen receptor; OCPs suppress LH secretion and hence reduce ovarian androgen production; and metformin improves insulin resistance. If the hirsutism is severe, all three medications may be employed. Patients need to be counselled that the symptoms of androgen excess (and hirsutism in particular) usually take at least six months to improve.
Eflornithine can be applied topically for rapid control of facial hirsutism, although fastidious use is necessary in order for the treatment to be effective. Antibiotics and retinoic acid derivatives can also be used for acne treatment. Permanent laser hair removal can be an effective treatment and should be considered if the symptoms are causing severe distress. Metformin is ineffective in controlling hirsutism in the majority of women.
Metabolic complications
Calorie restriction and exercise remain the mainstay of therapy for metabolic complications of PCOS. Metformin is beneficial for improving insulin sensitivity and can aid in weight loss. In patients who do not require fertility, statins can be used to treat dyslipidaemia. Bariatric surgery may be effective for patients with severe obesity, as the marked weight loss after the procedure usually resolves not only the metabolic disorders of PCOS but also PCOS itself, restoring ovulatory function and fertility.(10)
Infertility
For overweight and obese patients who are keen to conceive, the first-line treatment should be diet and exercise. This is particularly important in women who are preparing for pregnancy, in order to reduce the risk of complications such as gestational DM, preeclampsia, pre-term delivery, macrosomia, birth defects and stillbirth. Antiandrogens such as spironolactone should be stopped for three months before conception, and patients should be counselled about recurrence of androgen excess symptoms while preparing for fertility. Metformin may help, although it is unclear if this is independent of the weight loss benefit it confers. Ovulation induction with clomiphene citrate or letrozole is effective for fertility treatment. Monitoring the first cycle with ultrasonography can allow for dose titration and detection of multiple follicle development.(10)
WHEN SHOULD I REFER TO A SPECIALIST?
All patients with severe or rapidly progressive virilisation, or those with a testosterone level that is twice the upper limit of normal, should be referred to an endocrinologist or a gynaecologist to exclude other causes. If first-line measures for cycle control or androgen excess have failed, or are ineffective in controlling symptoms, then referral to an endocrinologist, gynaecologist or reproductive medicine specialist should be considered. Patients who desire fertility but are either anovulatory or have not conceived after six months of regular unprotected intercourse should be referred to a reproductive medicine department. Further specialist referral may be necessary for comorbidities such as severe obesity, DM, OSA and depression.
TAKE HOME MESSAGES
PCOS presents with a spectrum of conditions resulting from androgen excess, anovulation and metabolic syndrome.
Signs and symptoms of PCOS include hirsutism, acne, menstrual irregularities, infertility, obesity, and psychiatric disorders such as anxiety and depression.
Screening for T2DM, dyslipidaemia, hypertension, anaemia and vitamin D deficiency should be a consideration for all patients with PCOS.
Treatment for PCOS has to be targeted to each patient’s phenotype and personal expectations such as desire for pregnancy. Psychological well-being due to effects on physical appearance is also an important consideration.
Diet and exercise are major components in the management of patients with PCOS and obesity.
The first-line therapy for fertility and metabolic syndrome in PCOS is lifestyle modification with diet and exercise, followed by pharmacological therapy.
Patients with oligomenorrhoea have a risk of endometrial hyperplasia, and pharmacological therapy may be necessary to induce a withdrawal bleed if the interval between menses is longer than two months.
After six months on the oral contraceptive pill, you noted a great improvement in the condition of Andrea’s acne. She had also taken your advice to start regular exercise, which helped her to reduce her weight by 3 kg. Most importantly, she was focusing on being healthy instead of comparing herself with others.
REFERENCES
- 1.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841–55. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 3.Dramusic V, Goh VH, Rajan U, Wong YC, Ratnam SS. Clinical, endocrinologic, and ultrasonographic features of polycystic ovary syndrome in Singaporean adolescents. J Pediatr Adolesc Gynecol. 1997;10:125–32. doi: 10.1016/s1083-3188(97)70072-x. [DOI] [PubMed] [Google Scholar]
- 4.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X, Ni R, Li L, et al. Defining hirsutism in Chinese women: a cross-sectional study. Fertil Steril. 2011;96:792–6. doi: 10.1016/j.fertnstert.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Legro RS, Arslanian SA, Ehrmann DA, et al. Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indran IR, Huang Z, Khin LW, et al. Simplified 4-item criteria for polycystic ovary syndrome: a bridge too far? Clin Endocrinol (Oxf) 2018 May 30; doi: 10.1111/cen.13755. https://doi.org/10.1111/cen.13755. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman's long-term health using data linkage. J Clin Endocrinol Metab. 2015;100:911–9. doi: 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- 9.Deloche C, Bastien P, Chadoutaud S, et al. Low iron stores: a risk factor for excessive hair loss in non-menopausal women. Eur J Dermatol. 2007;17:507–12. doi: 10.1684/ejd.2007.0265. [DOI] [PubMed] [Google Scholar]
- 10.Conway G, Dewailly D, Diamanti-Kandarakis E, et al. ESE PCOS Special Interest Group. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171:P1–29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 11.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


