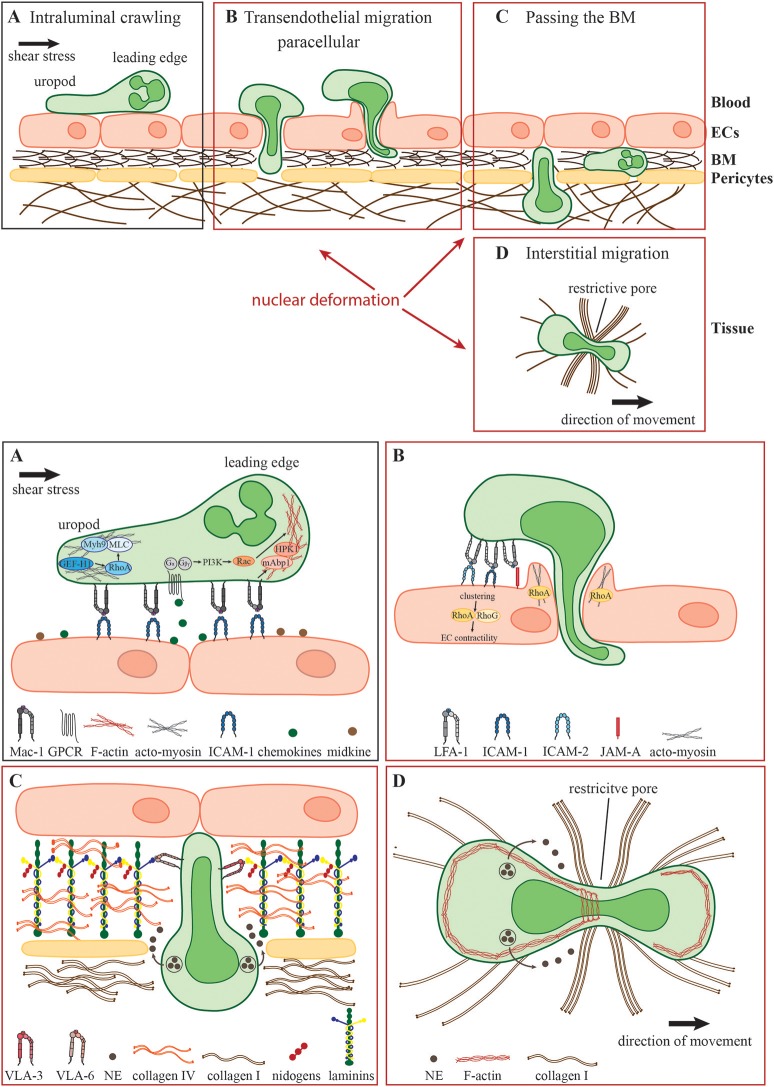
Figure 1.
Neutrophil migration in different environmental conditions. During the acute inflammatory response, (A) recently arrested neutrophils migrate along the inflamed endothelium (intraluminal crawling) toward potential exit sites. Intraluminal crawling is mainly mediated by the interaction of Mac-1 on neutrophils with ICAM-1 on inflamed ECs. Binding of chemokines and other chemoattractants to their respective GPCRs results in the dissociation of the Gα and Gβγ subunits with subsequent intracellular signaling inducing cell polarization with an F-actin-rich leading edge and an acto-myosin-rich uropod. (B) Neutrophils protrude and transmigrate through the endothelial monolayer via the paracellular route. Here, LFA-1- and Mac-1-engagement of endothelial ligands including ICAM-1, ICAM-2, and JAM-A activates endothelial Rho-GTPases e.g., RhoA, RhoG, and NMII leading to EC contractility and plasma leakage restriction. Neutrophils must use their own Rho GTPases to squeeze their nuclei through paracellular endothelial junctions triggering gap formation. (C) Following transmigration, neutrophils pass the subjacent basement membrane (BM) and often also crawl on adjacent pericytes embedded in the BM. The main components of the BM are laminins, collagen type IV and nidogens. Neutrophils penetrate this meshwork through LER which can be enlarged by the secretion of elastase (NE) and by nuclear squeezing, both potentially coordinated by the neutrophil integrins VLA-3 and VLA-6 and their interactions with BM collagens and laminins. (D) In the inflamed tissue, neutrophils migrate within a 3D collagen I-rich environment toward the site of inflammation (interstitial migration). Here, along with NE secretion, neutrophils deform and push forward their nuclei to pass through restrictive barriers in the meshwork of collagen fibers, most probably by dynamic interactions of their actin cytoskeleton with the nuclear lamina.