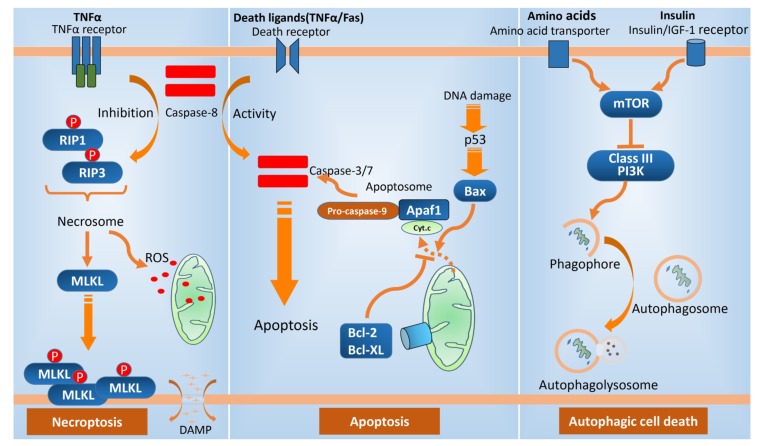
Fig. (1).
Three modes of programmed cell death. Apoptosis, a programmed form of nonlytic cell death, can induce the activation of effector caspases via a variety of upstream signals. In the so-called “intrinsic cell death pathway,” the P53 expression mediated by DNA damage triggers the production of Bax, a kind of pro-apoptotic protein, which can act to permeabilize the outer membranes of the mitochondria, releasing cytochrome c, which results in the oligomerization of an adapter protein, APAF1. APAF1 then binds to and activates the initiator caspase, caspase-9, and apoptosome forms. Bcl-2 protein family has anti-apoptotic activity. In the extrinsic cell death pathway, normally, caspase 8 can trigger apoptosis by classical caspase cascade. In the presence of caspase 8 inhibition, necroptosis is activated by the phosphorylation of RIP1 and RIP3, and then necrosome forms, which may activate the executioner of necroptosis, MLKL, leading to necroptosis. Autophagy can be induced in response to environmental signals including amino acids, hormones, and energy consumption. The best-characterized regulatory protein, mTOR, can act to inhibit autophagy. The Class III PI3K complex contributes to membrane expansion, resulting in the formation of a closed double-membrane structure, the autophagosome, which fuses with a lysosome to form an autolysosome.