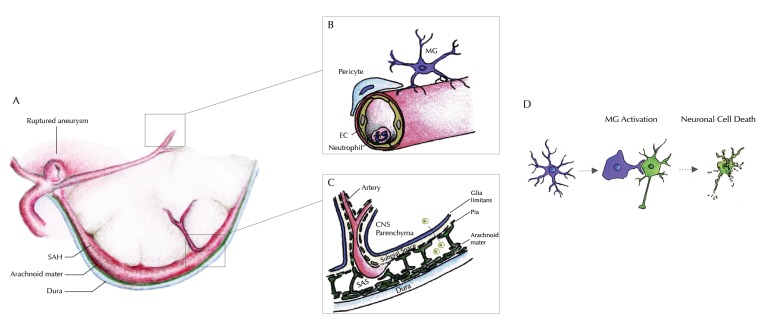
Fig. (1).
Illustration of our current understanding of inflammatory processes within the different compartments of the CNS. A: Rupture of an intracranial aneurysm within the subarachnoid space causes spilling of blood between the arachnoid mater and the pia mater – outside of the brain parenchyma. B: Within the cerebral microvessels, an increased gathering of neutrophil granulocytes at the endothelial surface is seen as a sign of intravascular inflammation, within the first 1-4 days after the bleeding. An extravasation (possibly into the subarachnoid space) has been reported, but transmigration into the brain parenchyma could be excluded. Around day 4 after the bleeding, an accumulation of microglia starts near the site of vessel rupture-i.e. near the large conductance vessels at the brain base. Knocking out of Neutrophil endothelial interaction inhibits microglia accumulation within the brain tissue. C: Signaling from the subarachnoid space into the brain parenchyma must therefore occur within the time window of vascular inflammation through the pia and the glia limitans. Profound understanding of the anatomical and molecular mechanisms of the blood brain barrier is therefore essential to understand this outside-in activation of innate immunity, that successively inflicts secondary brain injury. It remains unclear whether this signaling involves a cellular component (e.g. arachnoid lymphocytes) or occurs in a strictly non-cellular fashion. D: The inflammatory cascade continues through activation of innate immunity within the brain parenchyma. Microglia, the brain’s innate immune cells accumulate and are activated beginning from day four after the bleeding. Throughout the time interval of maximum accumulation (between days 9 and 14), microglia are also activated, expressing a variety of pro-inflammatory cytokines. Changing to their activated morphology, they gather around neurons, inflicting neuro-axonal injury, decimating the absolute number of surviving neurons significantly, hereby contributing substantially to secondary brain injury after SAH.