Abstract
The foreign body response (FBR) occurs ubiquitously to essentially all non-biological materials that are implanted into higher organisms. The FBR is characterized by inflammation followed by fibrosis and is mediated largely by macrophages. While many current medical devices tolerate the FBR, the FBR is responsible for many asceptic device failures and is hindering advancements of new devices that rely on device-host communication to function. To this end, in vitro and in vivo models are critical to studying how a biomaterial, via its chemistry and properties, affect the FBR. This short review highlights the main in vitro and in vivo models that are used to study the FBR. In vitro models that capture macrophage interrogation of a biomaterial and evaluation of macrophage attachment, polarization and fusion are described. In vivo models using rodents, which provide a relatively simple model of the complex FBR process, and human-relevant nonhuman primate models are described. Collectively, the combination of in vitro and in vivo models will help advance our fundmental understanding of the FBR and enable new biomaterials to be developed that can effectively modulate the FBR to achieve a desire device-host outcome.
Keywords: Macrophage, Foreign Body Response, Biomaterial, In vitro models, In vivo models
Graphical abstract
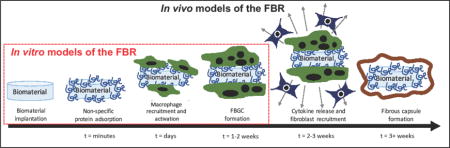
Introduction
Implantable medical devices have revolutionized medicine. Each year, millions of medical devices are implanted into patients leading to significant improvements in quality of life. For example, joint arthroplasty enables patients with severe osteoarthritis to return to an active lifestyle with minimal pain [1]. Cochlear implants provide patients with irreversible sensorineural hearing loss the ability to recognize speech and participate normally in social interactions [2]. However, all implantable devices suffer from complications. One problem that is ubiquitous to essentially all implantable devices, regardless of the synthetic or biologic nature of the device, is the foreign body response (FBR), the body’s normal response to a foreign material. While many medical devices that are currently implanted into humans function despite a FBR, this response has been linked to reported asceptic implant failures [3–5]. These failures have the potential to be devastating and create a significant burden on the healthcare system [6,7]. Moreover, advancements of new and more complex devices are hampered by the presence of a FBR. For example, the formation of a fibrous capsule can disrupt communication between host and device, which is important for the function of devices such as glucose sensors [8], islet transplantation [9], and tissue engineering scaffolds [10]. The FBR represents a formidable challenge to current and future implantable medical devices.
The FBR is characterized by chronic inflammation accompanied by the formation of a dense, avascular fibrous capsule [11,12]. The FBR begins with non-specific protein adsorption to the surface of the implant followed by the recruitment of inflammatory cells. The latter occurs as part of the initial injury response where neutrophils arrive first, but are soon replaced by long-lived macrophages, the orchestrators of the FBR. Macrophages recognize the implant as foreign through the adsorbed protein layer. Due to the implant size (i.e., much larger than foreign microorganisms), macrophages are unable to phagocytose the material and eventually fuse into foreign body giant cells (FBGCs). Concurrently, the FBR transitions to fibrous encapsulation, the hallmark of the FBR. Macrophages remain at the implant surface for the lifetime of the implant encased within the fibrous capsule and maintain low grade chronic inflammation. These events can have a detrimental impact on implanted medical devices. For example, long-term exposure to chronic inflammation induces corrosion or degradation of otherwise highly stable biomaterials [3,13]. Continuous exposure to inflammatory cytokines can negatively impact living cells within biomaterials that are implanted for islet transplantation or tissue engineering [14]. The fibrous capsule, which typically forms within three to four weeks after implantation, acts as an impenetrable wall to prevent communication between device and host tissue. Depending on the device, chronic inflammation and/or fibrous encapsulation can adversely affect the function of implantable medical devices.
Understanding how the FBR impacts the biomaterial and ultimately the function of the medical device is a critical step towards acheiving long-term in vivo success. In vitro and in vivo models that recapitulate aspects of the FBR offer powerful tools to study the FBR and its effect on the performance of a biomaterial. In vitro models reduce the complexity of the FBR. On the other hand, in vivo models capture the full temporal process of the FBR, which is still not completely understood. In vivo models are necessary to evaluate the formation of the fibrous capsule, the final stage of the FBR. Together, in vitro and in vivo models enable screening of new biomatierals to determine how they affect and are affected by the FBR. Moreover, these models can provide mechanistic insights into the processes that lead to the FBR, which will enable new and improved biomaterials to be developed. This short review summarizes the main in vitro and in vivo models that have been developed to assess the host response to implanted biomaterials and presents select key findings. Table 1 highlights these models along with their advantages and drawbacks.
Table 1.
Summary of models used for assessing the host response to biomaterials.
| Model | Advantages | Drawbacks | |
|---|---|---|---|
| In vitro models | Macrophage interrogation of a biomaterial | Enables assessment of macrophage activity as a function of biomaterial properties | Lacks influence of crosstalk between cell types present in the FBR in vivo |
| Co-culture of macrophages with other cell types | Allows for investigation of paracrine and juxtracrine signaling on macrophage activity | Limited to one phase of the FBR | |
| In vivo models | Rodent wild-type models | Supports assessment of the phases of the FBR over time | Limited assessment of redundant immune pathways |
| Genetically modified mouse models | Provides insight into the pathways and cell types that drive the FBR | Expensive | |
| Large animal models | Demonstrates translatability of mouse models; clinically relevant | Expensive; highly regulated |
In vitro models
In vitro models enable the investigation into discrete events associated with the FBR in a tightly controlled environment. These events include non-specific protein adsorption and macrophage attachment, polarization, and/or fusion into FBGCs (Fig. 1a). By culturing macrophages directly on a biomaterial surface, the role that surface chemistry and properties play on macrophage response can be studied. The following sections describe macrophage sources that are commonly used in vitro, assessment and induction of macrophage polarization and fusion, and the in vitro model representing macrophage interrogation of a biomaterial.
Figure 1.
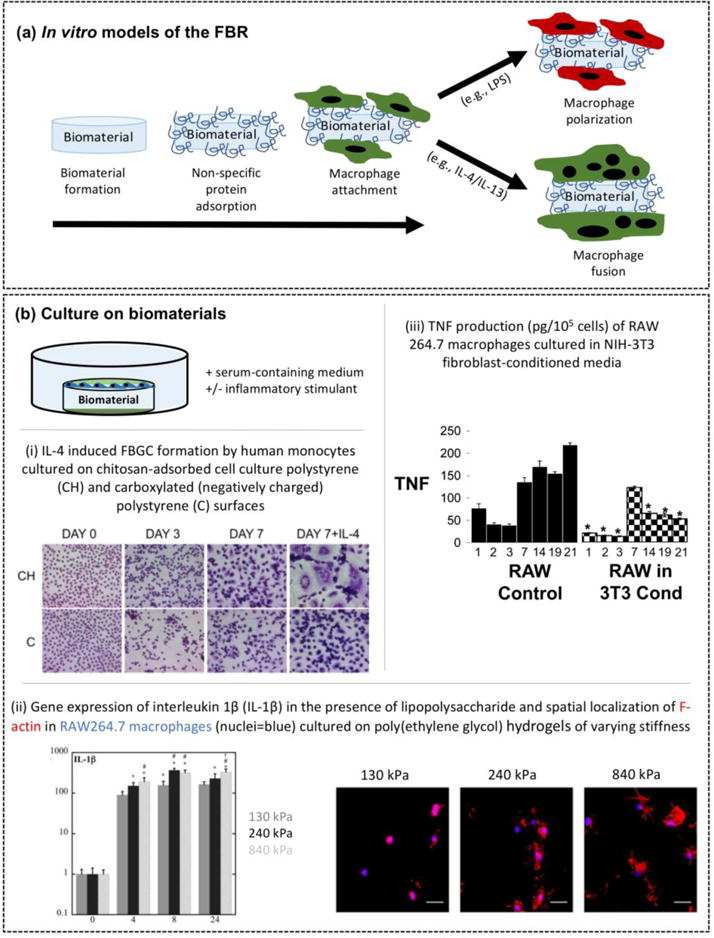
In vitro models of the FBR. (a) Schematic of in vitro models that capture specific events of the FBR by non-specific protein adsorption to the surface of an implanted biomaterial, macrophage attachment, macrophage activation via different polarization states and macrophage fusion into foreign body giant cells (FBGCs). (b) In vitro model that recapitulates macrophage interrogation of a biomaterial. Select results using the in vitro model. i) Differential interleukin-4 (IL-4)-induced foreign body giant cell (FBGC) formation by human monocytes cultured on two different substrates: chitosan-adsorbed onto cell culture polystyrene (CH) and carboxylated (negatively charged) polystyrene (C). Reproduced with permission from [32]. ii) Stiffness-dependent gene expression by RAW 264.7 macrophages for interleukin-1β (IL-1β) when cultured on poly(ethylene glycol) hydrogels in the presence of an inflammatory stimulant (lipopolysaccharide) after 0, 4, 8, and 24 hours of culture. Data show increased pro-inflammatory cytokine expression with increased stiffness. Spatial localization of F-actin (red) in RAW 264.7 macrophages (nuclei in blue) on poly(ethylene glycol) hydrogels highlights increased cell spreading with increasing stiffness. Reproduced with permission from [35]. iii) tumor necrosis factor (TNF) production (pg/105 cells) by RAW 264.7 macrophages was reduced when cultured in NIH3T3 fibroblast-conditioned media. Reproduced with permission from [36].
Macrophage source
Macrophages are recruited from blood-derived monoyctes and differentiate into macrophages as they migrate through the tissue to the implantation site. In vitro studies of the FBR have used monocyte/macrophage cell lines, blood-derived primary monocytes, and primary macrophages derived from either blood or bone marrow monocytes. The advantage of cell lines is their accessibility and straightforwardness to culture. Examples of murine macrophage cell lines include RAW 264.7, J774A.1, and IC-21, and human monocyte cell lines include THP-1 and U-937. The latter can be differentiated into macrophages. Primary monocytes or monocyte-derived macrophages have also been studied for their biologically relevance. However, they require human/animal subjects with institutional approval, can be time-intensive and expensive to isolate, and have limited passaging capacity. Several studies have compared cell lines to primary cell sources. For example, RAW 264.7, J774A.1 and IC-21 responded to inflammatory stimulants by increased expression of pro-inflammatory cytokines, which was similar to primary bone-marrow derived macrophages, but the magnitude was generally lower [15]. Contrarily, THP-1 monocytes were more responsive than human peripheral blood monocytes to biomaterial extracts and an exogenous inflammatory stimulant [16]. These studies suggest that cell lines and primary cells can provide useful information regarding the relative monocyte/macrophage response to different biomaterials in vitro. However, the magnitude of the response will depend on cell type and thus interpretation of the magnitude should be taken with caution.
Macrophage Polarization
To study the macrophage in the context of the FBR in vitro, identifying the state of macrophage polarization is important. Macrophages are often categorized by two distinct polarization states, classically activated (M1) macrophages, which are involved in inflammation, and alternatively activated (M2) macrophages, which are involved in non-inflammatory (i.e., regulatory and wound-healing) processes. However, macrophages exhibit a high degree of plasticity. A more appropriate characterization of macrophage polarization is to consider activation across a spectrum, where macrophages display charateristics that span multiple polarization states [17]. To this end, efforts to identify subtypes of M2 polarization states (i.e., M2a-M2d) based on the in vitro stimulant have also been described [18]. A summary of macrophage polarization states are presented in Table 2 including their activation, key surface markers, commonly secreted factors, and functional outcomes.
Table 2.
Macrophage polarization1
| Polarization State | Activation | Key Surface Markers and Secreted Factors | Functional Outcome |
|---|---|---|---|
| M1 | LPS, TNF-α, IFN-γ | CD86, MHC-II, TLR2, TLR4, iNOS, ROS, IL-12, IL-6 | • Phagocytosis of bacteria, debris, etc. • Inflammation |
| M2a | IL-4, IL-13 | CD163, MHC-II, Arginase-1,IL-1ra, IL-10, TGF-β, | • ECM production • Immunoregulation |
| M2b | Immune Complexes, LPS | CD86, MHC-II, IL-1, IL-6, IL-10, TNF-α | • Immunoregulation |
| M2c | IL-10, TGF-β | CD163, CD206, TLR1, TLR8, MMP-9, IL-10, TGF-β | • ECM production • FBGC formation and fibrosis • Wound healing |
| M2d | IL-6 | VEGF-A, IL-10, IL-12, TNF-α, TGF-β | • Immunoregulation |
Table constructed from [53–56]. Abbreviations: LPS lipopolysaccharide, TNF tumor necrosis factor, IFN interferon, IL interleukin, TLR toll-like receptor, MHC major histocompatibility complex, TGF transforming growth factor, iNOS inducible nitric oxide synthase, ROS reactive oxygen species, MMP matrix metalloproteinase, VEGF vascular endothelial growth factor.
Macrophage polarization can be mediated by the biomaterial itself, but is also confounded in vivo by the inflammatory environment that is associated with tissue injury at the time of implantation. The latter can be simulated in vitro by exogenous delivery of inflammatory stimulants such as lipopolysaccharide (LPS), a membrane component of gram-negative bacteria, and/or interferon gamma [19]. The inflammatory macrophage polarization state is most commonly characterized by NF-κB dependent transcription of pro-inflammatory cyotkines including TNF-α, IL-6, and IL-1β as well as other markers such as inducible nitric oxide (iNOS) [20]. The polarization state of alternatively activated macrophages is more complex to assess due to the different processes in which the macrophages are involved. Arginase type I, which converts arginine to orthinine, a precursor of collagen, has been used as a marker of a wound-healing polarization state [17,21]. Interleukin-10 has been used as a marker of a regulatory polarization state [17], which can inhibit the inflammatory state and act to self-regulate inflammation [22]. Several studies have demonstrated that macrophages display plasiticity in vitro whereby a shift in the polarization state from inflammatory to either wound-healing or regulatory phenotype has been reported [23,24].
A unqiue characteristic of macrophages in the FBR is their fusion into FBGCs, which are defined as having three or more nuclei per cell [25]. In vitro methods have been developed to induce macrophage fusion in vitro through the delivery of Th2 cytokines of interleukin-4 combined with either interleukin-13 or granulocyte macrophage colony-stimulating factor [26]. Using this protocol, fusion rates >70% can be achieved, compared to <10% without fusion mediators, and very lage FBGCs can be formed with >200 nuclei per cell [25]. Although fusion is more readily achieved in primary monocytes and macrophages, studies have reported that RAW264.7, THP-1 and U937 can undergo fusion, but the number of nuclei per FBGC is much lower [27].
Macrophage Interrogation of a Biomaterial
Macrophages interrogate implanted biomaterials through non-specifically adsorbed proteins. Proteins rapidly adsorb to all surfaces regardless of chemistry (e.g., hydrophobic and hydrophilic), but the amount, type and conformation of the adsorbed proteins will depend on surface chemistry [28–30]. Studies often use serum-containing medium or pre-treat the biomaterial in full serum prior to seeding with macrophages [30] to promote non-specific protein adsoprtion. While the mechanism by which the adsorbed proteins mediate the FBR is not well-understood, presence of these proteins at the surface is critical to emulating the FBR in vitro. In fact, studies have reported more than 200 unique proteins adsorbed to an implanted biomaterial [30].
To recapitulate the macrophage-biomaterial interactions, the in vitro model should consist of monocytes/macrophages seeded directly on top of a biomaterial in the presence of proteins (e.g., serum-containing medium) (Fig 1b). This in vitro model has enabled assessment of macrophage attachment, polarization state, and fusion as a function of biomaterial surface properties. For example, hydrophobic surfaces promote better attachment of monocytes and macrophages when compared to neutral hydrophilic surfaces, but the latter leads to greater activation of inflammatory macrophages [31]. In the presence of fusion mediators, FBGC formation is influenced by surface chemistry (Fig 1bi) [32]. Surface topography such as increased surface roughness [33], reduced alignment [27], and micron-sizes surfaces features [34] can enhance monocyte/macrophage attachment and/or activation to an inflammatory state. Inflammatory stimulants (e.g., LPS) can be used to simulate the inflammatory macrophage phenotype that is recruited to the implant site in vivo [15,23,35]. Studies have shown that LPS-mediated activation of inflammatory macrophages is dependent on the biomaterial properties, such as hydrogel stiffness (Fig 1bii) [35]. Thus, inflammatory stimulants combined with in vitro models can more closely approximate the in vivo environment to identify synergistic effects between biomaterial-type and macrophage activation and as well to identify biomaterials that are capable of attenuating macrophage activation.
In vitro models have also be adapted to investigate the impact of other cell types (e.g., fibroblasts and lymphocytes) in augmenting macrophage response in the FBR. Fibroblasts play an important role in the formation of the fibrous capsule. Studies have shown that paracrine factors secreted by fibroblasts, such as monocyte chemoattractant protein (MCP)-1, decrease pro-inflammatory cytokine production in macrophages (Fig 1biii) [36]. When fibroblast and monocyte were cultured together, secretion of CC chemokine ligand 2 (CCL2) was increased [33], which is a mediator of FBGC formation [37]. Together, these in vitro studies suggest that fibroblasts may play a role in facilitating the macrophage phenotypic switch from inflammation to fibrosis. Although the exact role of the adaptive immune system in the FBR is not well understood, studies report that monocyte/macrophage adhesion on biomaterial surfaces is decreased, but FBGC formation is increased, in the presence of lymphocytes [38]. These studies and others illustrate that paracrine signaling between macrophages and other cell types influences macrophage response to biomaterials and therefore may play an important role in the FBR in vivo.
In vivo models
In vivo models offer the “complete picture” of the FBR from inflammation to fibrosis (Fig 2a). This section highlights a) small animal rodent models (wildtype and genetically modified), which are commonly used to study the FBR and b) clinically relevant non-human primates, which have been used to study the FBR in animal models that more closely resemble humans.
Figure 2.
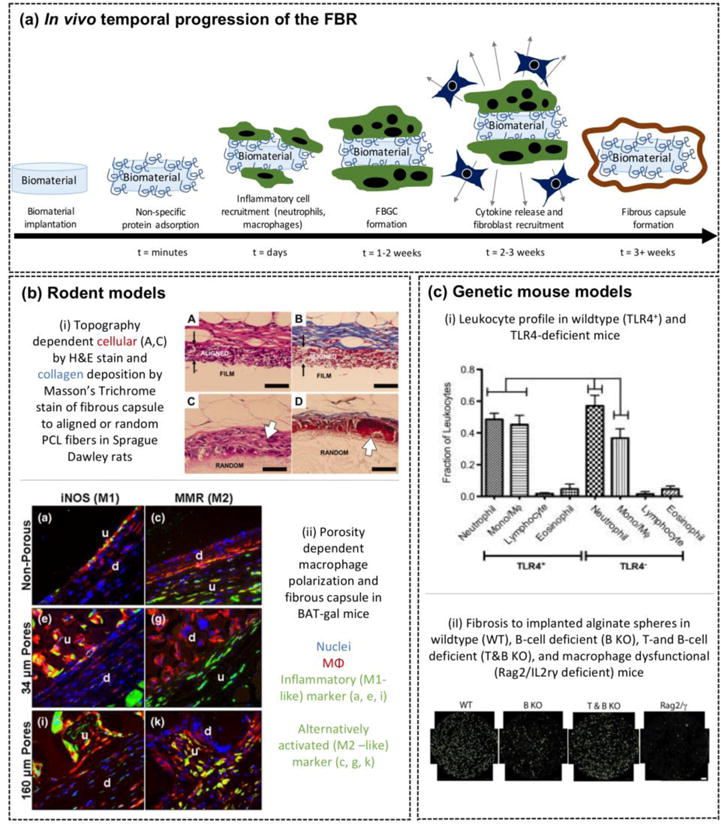
In vivo models of the FBR. (a) Schematic of the temporal progression of the FBR in vivo, characterized by biomaterial implantation followed by immediate non-specific protein adsorption, inflammatory cell recruitment, macrophage fusion into foreign body giant cells (FBGCs) and resolving in fibrous capsule formation. (b) Select results using the in vivo rodent models. i) Topography-dependent fibrous capsule formation to subcutaneous implantation of aligned or random PCL fibers in Sprague Dawley rats. H&E staining shown on the left and Masson’s Trichrome staining shown on the right. Open arrows in panels C and D show macrophage presence and FBGC formation. Reproduced with permission from [44]. ii) Porosity dependent macrophage polarization and fibrous capsule formation in BAT-gal mice. Macrophages shown in red with nuclei in blue. Green indicates an inflammatory macrophage polarization state (iNOS) or an alternatively activated macrophage polarization state (MMR, a marker of fusion). Reproduced with permission from [45]. (c) Select results using the in vivo genetic mouse models to elucidate pathways within the FBR. i) Differential leukocyte profile in WT and TLR4-defficient mice following PET disc implantation. Reproduced with permission from [49]. ii) Fibrosis to alginate microspheres was reduced in B-cell deficient mice, restored in T-and B-cell deficient mice, and further reduced in MΦ-dysfunctional (Rag2/γ) mice. Reproduced with permission from [50].
Rodent wild-type models
The most common in vivo model of the FBR is implantation at subcutaneous or intraperitoneal sites in mice or rats. Several studies have shown differences in the FBR with implantation site, where the intraperitoneal site had higher levels of proangiogenic factors and lower levels of pro-inflammatory cytokines during the initial stage of the FBR [39]. However, both sites led to fibrous encapsulation [36,37]. The former is attributed to the faster healing response that occurs in the periotenal cavity compared to the dermis [40]. The mouse strain also affects the FBR with the C57BL/6 strain showing a more robust FBR and fibrous encapsulation mimicking that of humans when compare to the BABL/c strain [41]. Using rodent models, studies have identified differences in the FBR based on biomaterial chemistry and properties. For example, macrophage apoptosis was higher on hydrophilic and anionic biomaterials compared to hydrophobic biomaterials [42]. Macrophage accumulation was higher with increasing stiffness of neutral hydrogels, a finding that mirrored parallel in vitro studies, which are shown in Fig 1bii [35]. Zwitterionic hydrogels, on the other hand, substantially reduced fibrous encapsulation compared to neutral hydrogels [43]. Morover, nanofibrous scaffolds that were aigned resulted in significantly thinner fibrous capsule when compared to random scaffolds (Fig 2bi) [44]. Similarly, porosity influenced macrophage polarization and fibrous capsule formation (Fig 2bii) [45]. Smaller pores (34 μm) led to an alternatively activated macrophage phenotype at the implant surface and a thinner capsule while larger pores (160 μm) led to inflammatory macrophages and a thicker capsule. Assessment of protein expression in macrophages and FBGCs adhered to an implant revealed the presence of cytokines, interleukin-4 and interleukin-13, and the growth factor TGF-β [46], which are consistent fibrosis [18]. Collectively, these studies and many more demonstrate that while a FBR occurs to nearly all implanted non-biological biomaterials, the severity of the FBR depends on the nature of the surface.
Genetically modified mouse models
Genetically modifed mouse models provide the opportunity to gain insight into the cell types and/or pathways that mediate the FBR. For example, biomaterial implantation in mice deficient in T-cells [38], natural killer cells [47], or mast cells [47,48] demonstrated normal formation of FBGCs and/or fibrous capsule formation, suggesting these inflammatory cells are not essential to the FBR [41]. Mice lacking toll-like receptor-4, a cell suface receptor that recognizes damage-associated molecular patterns, led to a shift in the recruited leukocyte profile with fewer monocytes/macrophages (Fig 2ci), but this shift was insufficient to affect fibrous capsule formation [49]. Mice lacking Rag2 and IL2rγ, which leads to macrophage dysfunction, resulted in no observable fibrosis at day 14 (Fig 2cii) [50]. Collectively, implant studies with genetically modified mouse models have confirmed that macrophages are critical to the FBR and the formation of the fibrous capsule. While other immune cells may have a role in the FBR (e.g., via cytokine secretion), the immune system has built in redundancies such that signals involved in the FBR most likely arise from more than one cell type.
Large animal models
Pre-clinical large animal models are needed to bridge the knowledge gap between rodent models and humans. Nonhuman primates (NHP), in particular, closely mimic the human immunology [51] and therefore provide an important step towards understanding the FBR in humans. Several studies have investigated the FBR to implanted biomaterials [50,52]. For example, studies have shown that biomaterials implanted in NHP lead to a dense fibrous encapsulation and the presence of FBGCs similar to that observed in C57BL/6 mice [50]. In addition, alterations in biomaterial chemistry, which reduce the fibrotic response in C57BL/6 mice, similarly reduce the FBR in NHP [52]. Morover, assessment of immune factors within the implant region were similar to those identified in the C57BL/7 mice [50]. Collectively, these studies suggest that findings from mouse models are translatable to clinically relevant large animal models and therefore can serve as a sutiable model prior to testing in NHP.
Concluding Remarks
The FBR continues to present a formidable challenge to implantable devices. For some devices (e.g., glucose sensors, islet transplantation) complete abrogation of the FBR is desired, but for other devices (e.g., joint arthroplasty, tissue engineering scaffolds, etc.) modulating the FBR to a normal wound healing process is necessary for device-host integration. In the latter, inflammatory macrophages will be necessary, but their presence must be short-lived. In vitro models utilizing macrophage interrogation of the biomaterial combined with in vivo relevant cytokines can provide key mechanistic insights into the role of the biomaterial in mediating macrophage response. In vivo models are critical for assessing the fibrotic response, which is difficult to simulate in vitro. Collectively, in vitro models when combined with in vivo models will help advance our fundmental understanding of the FBR and enable new biomaterials to be developed that can effectively modulate the FBR to achieve a desire device-host outcome.
Acknowledgments
This work was in part supported by supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21AR064436. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also acknowledge support from a Department of Education GAANN fellowship to LSS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. Bmj Open. 2012;2:e000435. doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roche JP, Hansen MR. On the Horizon Cochlear Implant Technology. Otolaryngol Clin North Am. 2015;48:1097–+. doi: 10.1016/j.otc.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibon E, Cordova LA, Lu L, Lin T-H, Yao Z, Hamadouche M, et al. The biological response to orthopedic implants for joint replacement. II: Polyethylene, ceramics, PMMA, and the foreign body reaction. J Biomed Mater Res Part B-Appl Biomater. 2017;105:1685–91. doi: 10.1002/jbm.b.33676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Malley JT, Burgess BJ, Galler D, Nadol JB. Foreign Body Response to Silicone in Cochlear Implant Electrodes in the Human. Otol Neurotol. 2017;38:970–7. doi: 10.1097/MAO.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT (TM) in pediatric patients. Eur J Cardiothorac Surg. 2003;23:1002–6. doi: 10.1016/S1010-7940(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 6.Tateishi M, Tomizawa Y. Intravascular foreign bodies: danger of unretrieved fragmented medical devices. J Artif Organs. 2009;12:80–9. doi: 10.1007/s10047-009-0447-6. [DOI] [PubMed] [Google Scholar]
- 7.Heneghan C, Thompson M, Billingsley M, Cohen D. Medical-device recalls in the UK and the device-regulation process: retrospective review of safety notices and alerts. Bmj Open. 2011;1:e000155. doi: 10.1136/bmjopen-2011-000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398:1695–705. doi: 10.1007/s00216-010-4097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper DKC, Matsumoto S, Abalovich A, Itoh T, Mourad NI, Gianello PR, et al. Progress in Clinical Encapsulated Islet Xenotransplantation. Transplantation. 2016;100:2301–8. doi: 10.1097/TP.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crupi A, Costa A, Tarnok A, Melzer S, Teodori L. Inflammation in tissue engineering: The Janus between engraftment and rejection. Eur J Immunol. 2015;45:3222–36. doi: 10.1002/eji.201545818. [DOI] [PubMed] [Google Scholar]
- 11.Ratner BD, Bryant SJ. Biomaterials: Where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 13.Gibon E, Amanatullah DF, Loi F, Pajarinen J, Nabeshima A, Yao Z, et al. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J Biomed Mater Res Part B-Appl Biomater. 2017;105:2162–73. doi: 10.1002/jbm.b.33734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swartzlander MD, Lynn AD, Blakney AK, Kyriakides TR, Bryant SJ. Understanding the host response to cell-laden poly(ethylene glycol)-based hydrogels. Biomaterials. 2013;34:952–64. doi: 10.1016/j.biomaterials.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamberlain LM, Godek ML, Gonzalez-Juarrero M, Grainger DW. Phenotypic non-equivalence of murine (monocyte-) macrophage cells in biomaterial and inflammatory models. J Biomed Mater Res A. 2009;88A:858–71. doi: 10.1002/jbm.a.31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heil TL, Volkmann KR, Wataha JC, Lockwood PE. Human peripheral blood monocytes versus THP-1 monocytes for in vitro biocompatibility testing of dental material components. J Oral Rehabil. 2002;29:401–7. doi: 10.1046/j.1365-2842.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- 17*.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klopfleisch R. Macrophage reaction against biomaterials in the mouse model – Phenotypes, functions and markers. Acta Biomater. 2016;43:3–13. doi: 10.1016/j.actbio.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci-Landmark. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 21.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, et al. Arginase-1-Expressing Macrophages Suppress Th2 Cytokine-Driven Inflammation and Fibrosis. Plos Pathog. 2009:5. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore KW, Malefyt RD, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 23.Lynn AD, Bryant SJ. Phenotypic changes in bone marrow-derived murine macrophages cultured on PEG-based hydrogels activated or not by lipopolysaccharide. Acta Biomater. 2011;7:123–32. doi: 10.1016/j.actbio.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamberlain LM, Holt-Casper D, Gonzalez-Juarrero M, Grainger DW. Extended culture of macrophages from different sources and maturation results in a common M2 phenotype. J Biomed Mater Res A. 2015;103:2864–74. doi: 10.1002/jbm.a.35415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Anderson JM. Multinucleated giant cells. Curr Opin Hematol. 2000;7:40–7. doi: 10.1097/00062752-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 26.McNally AK, Anderson JM. Interleukin-4 Induces Foreign-Body Giant-Cells From Human Monocytes Macrophages -Differential Lymphokine Regulation Of Macrophage Fusion Leads To Morphological Variants Of Multinucleated Giant-Cells. Am J Pathol. 1995;147:1487–99. [PMC free article] [PubMed] [Google Scholar]
- 27.Jay SM, Skokos EA, Zeng J, Knox K, Kyriakides TR. Macrophage fusion leading to foreign body giant cell formation persists under phagocytic stimulation by microspheres in in vitro and in vivo in mouse models. J Biomed Mater Res A. 2010;93:189–99. doi: 10.1002/jbm.a.32513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratner Buddy D, Hoffman Allan S, Schoen Fredrick J, Lemon Jack E. Biomaterials Science: An Introduction to Materials in Medicine. Third. New York, NY: Academic Press; 2012. [Google Scholar]
- 29.Faulon Marruecos D, Kastantin M, Schwartz DK, Kaar JL. Dense Poly(ethylene glycol) Brushes Reduce Adsorption and Stabilize the Unfolded Conformation of Fibronectin. Biomacromolecules. 2016;17:1017–25. doi: 10.1021/acs.biomac.5b01657. [DOI] [PubMed] [Google Scholar]
- 30.Swartzlander MD, Barnes CA, Blakney AK, Kaar JL, Kyriakides TR, Bryant SJ. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials. 2015;41:26–36. doi: 10.1016/j.biomaterials.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, et al. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83A:585–96. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 32*.McNally AK, Anderson JM. Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophages in vitro: dependence on material surface properties. J Biomed Mater Res A. 2015;103:1380–90. doi: 10.1002/jbm.a.35280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zickus C, Kunkel SL, Simpson K, Evanoff H, Glass M, Strieter RM, et al. Differential regulation of C-C chemokines during fibroblast–monocyte interactions: adhesion vs. inflammatory cytokine. pathways. 1998;7:269–74. doi: 10.1080/09629359890956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen SL, Jones JA, Xu YG, Low HY, Anderson JM, Leong KW. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials. 2010;31:3479–91. doi: 10.1016/j.biomaterials.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100A:1375–86. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Skokos EA, Charokopos A, Khan K, Wanjala J, Kyriakides TR. Lack of TNF-alpha-Induced MMP-9 Production and Abnormal E-Cadherin Redistribution Associated with Compromised Fusion in MCP-1-Null Macrophages. Am J Pathol. 2011;178:2311–21. doi: 10.1016/j.ajpath.2011.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyriakides TR, Foster MJ, Keeney GE, Tsai A, Giachelli CM, Clark-Lewis I, et al. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165:2157–66. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez A, MacEwan SR, Meyerson H, Kirk JT, Anderson JM. The foreign body reaction in T-cell-deficient mice. J Biomed Mater Res A. 2009;90A:106–13. doi: 10.1002/jbm.a.32050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendes JB, Campos PP, Ferreira MAND, Bakhle YS, Andrade SP. Host response to sponge implants differs between subcutaneous and intraperitoneal sites in mice. J Biomed Mater Res B Appl Biomater. 2007;83:408–15. doi: 10.1002/jbm.b.30810. [DOI] [PubMed] [Google Scholar]
- 40.Chegini N. Peritoneal molecular environment, adhesion formation and clinical implication. Front Biosci. 2002;7:E91–115. doi: 10.2741/chegini. [DOI] [PubMed] [Google Scholar]
- 41.King A, Sandler S, Andersson A. The effect of host factors and capsule composition on the cellular overgrowth on implanted alginate capsules. J Biomed Mater Res. 2001;57:374–83. doi: 10.1002/1097-4636(20011205)57:3<374∷AID-JBM1180>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 42.Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, et al. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc Natl Acad Sci U S A. 2002;99:10287–92. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Cao ZQ, Bai T, Carr L, Ella-Menye JR, Irvin C, et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol. 2013;31:553–7. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 44*.Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010;93:1151–9. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann Biomed Eng. 2014;42:1508–16. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- 46.Higgins DM, Basaraba RJ, Hohnbaum AC, Lee EJ, Grainger DW, Gonzalez-Juarrero M. Localized Immunosuppressive Environment in the Foreign Body Response to Implanted Biomaterials. Am J Pathol. 2009;175:161–70. doi: 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Jao B, McNally AK, Anderson JM. In vivo quantitative and qualitative assessment of foreign body giant cell formation on biomaterials in mice deficient in natural killer lymphocyte subsets, mast cells, or the interleukin-4 receptora and in severe combined immunodeficient mice. J Biomed Mater Res A. 2014;102:2017–23. doi: 10.1002/jbm.a.35152. [DOI] [PubMed] [Google Scholar]
- 48.Avula MN, Rao AN, McGill LD, Grainger DW, Solzbacher F. Foreign body response to subcutaneous biomaterial implants in a mast cell-deficient Kitw-Sh murine model. Acta Biomater. 5(10):1856–63. doi: 10.1016/j.actbio.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 49*.Rogers TH, Babensee JE. Altered adherent leukocyte profile on biomaterials in Toll-like receptor 4 deficient mice. Biomaterials. 2010;31:594–601. doi: 10.1016/j.biomaterials.2009.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, et al. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat Mater. 2017;16:671–80. doi: 10.1038/nmat4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Messaoudi I, Estep R, Robinson B, Wong SW. Nonhuman Primate Models of Human Immunology. Antioxid Redox Signal. 2011;14:261–73. doi: 10.1089/ars.2010.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34:345–+. doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rőszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015 doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duluc D, Delneste Y, Tan F, Moles M-P, Grimaud L, Lenoir J, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–30. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 55.Gordon S, Martinez FO. Alternative Activation of Macrophages: Mechanism and Functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]


