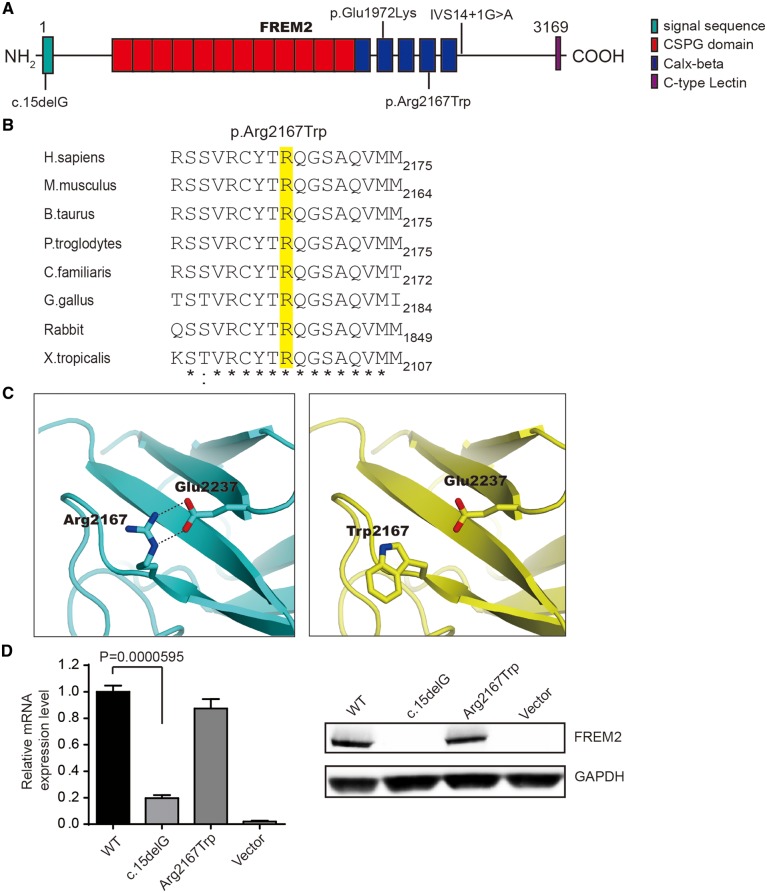
Figure 2.
Characterization of identified novel mutations in FREM2. (A) A schematic of the FREM2 protein and mutations in FREM2 (reported, upside; identified, downside). The functional domains are indicated by colored boxes. (B) Evolutionary conservation of the Arg2167 residue (highlighted in yellow) in FREM2 across species. (C) The expression of mutants was assessed by q-PCR (left panel) and WB (right panel). Results of q-PCR were presented as means±SEM from six independent experiments. (D) Three-dimensional structure of the Calx-beta domain with the missense mutation site Arg2167 in FREM2. A salt bridge between Arg2167 and Glu2237 was disrupted by the mutation p.Arg2167Trp.