Synopsis
Context is critical to the adaptive value of communication. Sensory systems such as the auditory system represent an important juncture at which information on physiological state or social valence can be added to communicative information. However, the neural pathways that convey context to the auditory system are not well understood. The serotonergic system offers an excellent model to address these types of questions. Serotonin fluctuates in the mouse inferior colliculus (IC), an auditory midbrain region important for species-specific vocalizations, during specific social and non-social contexts. Furthermore, serotonin is an indicator of the valence of event-based changes within individual social interactions. We propose a model in which the brain’s social behavior network serves as an afferent effector of the serotonergic dorsal raphe nucleus in order to gate contextual release of serotonin in the IC. Specifically, discrete vasopressinergic nuclei within the hypothalamus and extended amygdala that project to the dorsal raphe are functionally engaged during contexts in which serotonin fluctuates in the IC. Since serotonin strongly influences the responses of IC neurons to social vocalizations, this pathway could serve as a feedback loop whereby integrative social centers modulate their own sources of input. The end result of this feedback would be to produce a process that is geared, from sensory input to motor output, toward responding appropriately to a dynamic external world.
Introduction
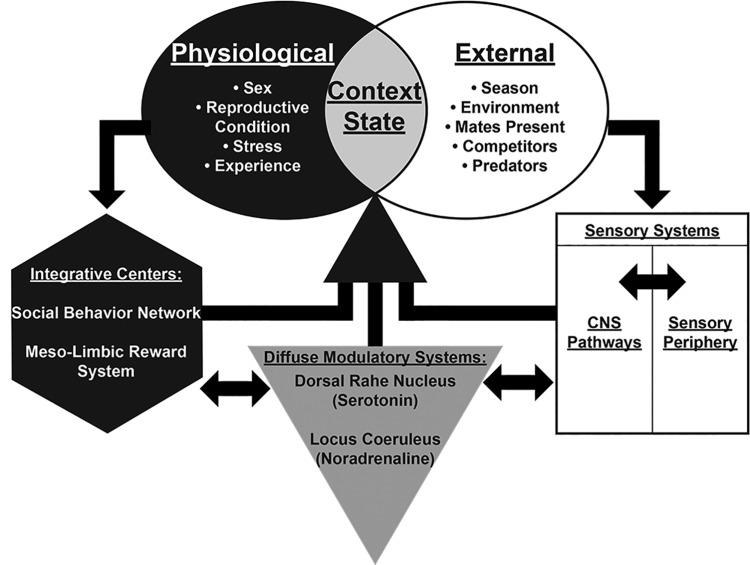
The ability for an animal to contextualize communication signals has strong fitness consequences. For instance, an animal in a reproductive stage of its cycle may be responsive to courtship signals produced by potential mates (Chakraborty and Burmeister 2009; Forlano and Bass 2011; Maney and Pinaud 2011). However, identical cues received in a non-reproductive phase (Maney etal. 2006; Lynch and Wilczynski 2008; Maney etal. 2008) or in the presence of a predator (Bernal etal. 2007; Grimsley etal. 2013) might be perceived as less salient, and fail to elicit a behavioral response. Therefore, an animal’s contextual state, which comprises the interaction between external events or context and an animal’s physiological state and past experience, contributes to the adaptive value of communication (Maney 2013) (Fig. 1). Sensory systems play an important role in this process by transforming the physical components of external cues (i.e., sound waves, odorants, etc.) into meaningful neural representations that are critical to the assessment of social signals. Sensory systems are also the first stage at which cues from the external environment can be coordinated with internal representations of state or salience. There is ample evidence that sensory systems are responsive to contextual state on time scales related to both predictable seasonal changes that induce concomitant changes in internal physiology (Caras 2013; Forlano etal. 2015), and to the more unpredictable dynamics of social interactions (Remage-Healey etal. 2008; Hall etal. 2011; Keesom and Hurley 2016). Furthermore, context- and state-dependent sensory processing contributes to the behavioral outcomes of social interactions (Lynch and Wilczynski 2008; Grimsley etal. 2013; Marlin etal. 2015). Despite many examples of these phenomena, the neural pathways that connect brain regions that evaluate external context and physiological state with sensory systems are not clear.
Fig. 1.
The emergence of contextual state. An animal’s contextual state is established by a complex interaction between internal physiology, environmental conditions, and the neural circuitry sensitive to both.
Here, we discuss studies demonstrating that sensory systems are sensitive to changes in internal state and external circumstances, which facilitate the ability for an animal to respond appropriately to socially relevant sensory cues. Next, we review work from our lab demonstrating that the neuromodulator serotonin is one mechanism through which contextual state may be conveyed to the auditory system. Finally, we present evidence in support of an emerging model of centralized neuromodulatory systems such as the serotonergic system as a link between neurochemical systems nested within social behavior circuits (e.g., the nonapeptides oxytocin and vasopressin) and primary sensory systems.
Salient states and events influence sensory systems
Long-term shifts in the response characteristics of sensory systems are one way that animals can adapt to predictable changes in internal state, such as during the reproductive phases of seasonal or cyclical breeders. For example, steroid hormones organize peripheral sensory systems to preferentially encode social signals that are salient only with respect to an animal’s reproductive condition. Female midshipman fish (Porichthys notatus) depend on their auditory systems to locate the nests of vocally courting males during annual spawning seasons (Brantley and Bass 1994). The inner ears of reproductive females are more sensitive to the higher frequencies of male vocalizations than those of non-reproductive females, a mechanism that is thought to aid in sound source localization (Sisneros and Bass 2003). Treating non-reproductive females with testosterone or estradiol induces a reproductive auditory phenotype, suggesting that seasonal fluctuations of steroid hormones induce auditory plasticity that is both adaptive and a signal of reproductive state (Sisneros etal. 2004; Coffin etal. 2012). In an analogous circumstance, female mice (Mus musculus) which rely heavily on olfactory signaling for reproduction and survival, become “smell blind” to male urine during diestrus, a non-reproductive phase of the estrous cycle (Dey etal. 2015). During diestrus, progesterone silences a subset of vomeronasal sensory neurons (VSNs) that bind behaviorally salient male urinary proteins, which in turn reduces female preference for male urine. Importantly however, VSNs that bind ligands within cat urine remain stable throughout the estrous cycle (Dey etal. 2015). Changes in the sensitivity of peripheral sensory systems are therefore not only confined to reproductive phases, but are also selective for reproductively relevant stimuli.
On event-related time scales, neuromodulators such as the nonapeptides oxytocin and vasopressin, which are nested within functionally heterogeneous social behavior circuits, are engaged to influence sensory systems (Caldwell and Young 2006; Albers 2015; Bester-Meredith etal. 2015). Sensory modulation also occurs via centralized integrative centers like the serotonergic dorsal raphe nucleus (DRN) or the noradrenergic locus coeruleus (Hurley etal. 2004). Despite differences in neural architecture, developmental trajectories, and evolutionary histories (Jacobs and Azmitia 1992; Stoop etal. 2015), these systems show strong functional parallels in their regulation of sensory information. Modulatory neurons strongly respond to external events, and further may respond best to specific qualities of these events indicating behavioral relevance (Bharati and Goodson 2006; Ho etal. 2010; Petersen etal. 2013; Dass and Vyas 2014; Kelly and Goodson 2015). As a result, these modulatory pathways can alter the responses of sensory neurons to stimuli. For example, chemically ablating noradrenergic neurons abolishes the selectivity of transcriptional activation by conspecific versus heterospecific songs in auditory forebrain regions of female canaries (Serinus canaria) (Lynch and Ball 2008). Similarly, ablating noradrenergic neurons in female canaries reduces copulatory responses to otherwise salient male courtship songs (Appeltants etal. 2002). Although neuromodulatory pathways broadly coordinate responses to salient events across neural systems (Lee etal. 2008; Mitre etal. 2016; Smith etal. 2017), only a few studies have directly addressed the influence of modulation within sensory regions on behavior. For example, locally increasing oxytocin within the auditory cortex of virgin female mice causes pup retrieval behavior to develop more rapidly than for control treatments (Marlin etal. 2015). Although these studies demonstrate a key role for sensory modulation in altering the behavior of receivers, the pathways that lead to release of neuromodulators within sensory systems are not well understood.
Sensory regions receive direct projections from modulatory systems. In rodents, the inferior colliculus (IC), an auditory midbrain structure important for processing species-specific vocalizations, receives the vast majority of its serotonergic innervation from the DRN (Klepper and Herbert 1991). Serotonin increases within the IC of mice during social or stressful encounters and its release is thought to be indicative of the salience of external conditions (see below). However, the DRN is not embedded within the brain’s social behavior network (SBN) (Jacobs and Azmitia 1992). Rather, “top-down” mechanisms are required to recruit DRN neurons in a context-dependent manner (Challis and Berton 2015). The DRN receives projections from over 100 functionally distinct nuclei including each node of the SBN (Pollak Dorocic etal. 2014; Weissbourd etal. 2014). The DRN’s afferent profile may provide it with the flexibility to convey features of social and non-social context to sensory systems, including the IC. Likewise, the DRN’s extensive projections make it highly suitable to distribute these features to wide array of brain regions (Jacobs and Azmitia 1992).
Serotonin release in IC is context dependent and valence sensitive
The IC is an obligate gate through which most ascending auditory information from the brainstem must pass (Winer and Schreiner 2005). Due to the combination of inhibitory and excitatory inputs from lower auditory areas, acoustic responses are more selective to species-specific calls within the IC than in its brainstem afferents (Bauer etal. 2002; Klug etal. 2002; Xie etal. 2005). In addition to receiving projections from the auditory thalamus (Senatorov and Hu 2002) and cortex (Xiong etal. 2015), IC receives neuromodulatory afferents from serotonergic, noradrenergic, and dopaminergic neural populations (Klepper and Herbert 1991; Hurley and Thompson 2001; Nevue etal. 2015), which can potentially convey information on contextual state to auditory regions. To demonstrate that neuromodulation within the IC is indicative of contextual state, it is necessary to use a measurement technique that captures dynamic fluctuations in modulator levels on timescales relevant to salient events like social interactions. Our lab uses carbon-fiber voltammetry to monitor invivo changes of the neuromodulator serotonin in freely behaving laboratory mice (CBA/J; Jackson Labs). This technique provides us with an invivo assay to measure how levels of serotonin fluctuate within IC while mice navigate various social and non-social contexts
Serotonin release in the IC is dependent on external events. Within 5 min after the onset of restriction stress, serotonin not only increases in the IC of male and female mice, but is also maintained at an elevated level throughout the duration of restriction (Fig. 2) (Hall etal. 2010; Hall etal. 2012; Hanson and Hurley 2014). Similarly, exposure to broadband noise elicits a rapid increase of serotonin in the IC of male mice that is sustained over the course of the stimulus (Fig. 2) (Hall etal. 2010). However, no such increase is observed when mice are presented with food or light stimuli (Fig. 2) (Hall etal. 2010), each of which has been demonstrated to affect levels of Fos, an immediate early gene product and putative marker for neural activation, (Clayton 2000) within serotonergic neurons in DRN (Hale etal. 2008; Takase and Nogueira 2008). Interestingly, exposure to TMT (2,5-dihydro-2,4,5-trimethylthiazoline; a component of fox urine) increases levels of serotonin in dialysate obtained from the prefrontal cortex and central amygdala (Smith etal. 2006), but not during voltammetric recordings within IC (Hall etal. 2010). A direct comparison between these studies is difficult as different methods (i.e., voltammetry vs. microdialysis) capture distinct aspects of serotonergic signaling. In particular, voltammetry with large carbon fibers likely captures a “volume transmission” mode of serotonin release, rather than more localized synaptic events (Bunin and Wightman 1998). Regardless, it is intriguing that a salient cue such as predator odor does not affect voltammetrically-measured serotonin in the IC given that similar cues (i.e., cat fur) reduce behavioral preference for female vocalizations in male mice (Grimsley etal. 2013). Together these studies show that not every change in a mouse’s external context is sufficient to increase levels of serotonin in IC, nor does serotonin release in IC appear to be an indicator of generally aversive contexts. Rather, serotonin increases in IC may accompany non-social contexts in which auditory processing is important, such as noisy conditions or restricted environments in which acoustically locating conspecifics may lead to escape.
Fig. 2.
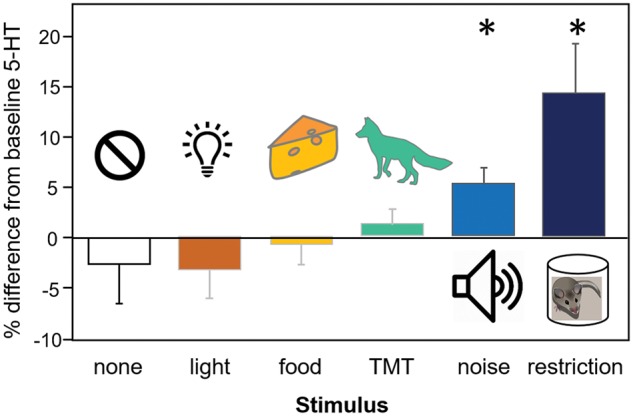
Serotonergic increases within the IC are dependent on specific external events. Of the five non-social conditions tested, only the presentation of noise and the restriction of movement within a cylindrical arena significantly increased serotonin relative to no stimulus. Figure adapted from Hall etal. 2010, J Exp Bio 213:1009–1017.
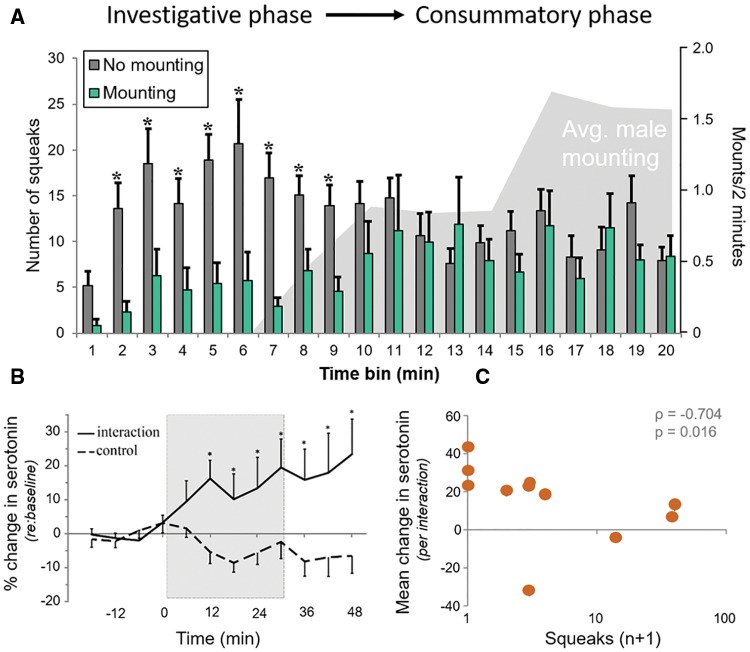
Mice use vocal communication during social encounters, making conspecific interactions a prime candidate for modulation of auditory information by contextual state. Male mice modulate the production of ultrasonic courtship vocalizations depending on the estrous state of their female social partner (Hanson and Hurley 2012). Female mice emit audible broadband vocalizations that may have positive or negative valence for males depending on when over the time course of a social interaction they are emitted (Finton etal. 2017). While the exact information that these social vocalizations carry remains unknown, we would predict that serotonin increases in the IC during social encounters in order to processes salient vocal acoustic cues. This is indeed the case. During social interactions with novel, opposite sex conspecifics, male (Fig. 3b) and female mice have increased IC serotonin relative to isolated controls (Hanson and Hurley 2014; Keesom and Hurley 2016). In contrast to non-social contexts in which serotonin increases relatively quickly (<5 min), quantitative differences in IC serotonin are not observed in opposite sex interactions until at least 12 min after the introduction of a social partner. Serotonin also increases over a similar time scale within the IC of males in direct social contact with a novel male (Hall etal. 2011). The presence of a social partner, however, is insufficient to gate serotonin release into IC, as focal males show no such increase when same sex interactions occur through a perforated barrier (Hall etal. 2011). This suggests that serotonin release in the IC is achieved through a particular combination of multi-modal cues. Finally, serotonin levels fluctuate relative to both self-generated and received behaviors. Across male-male interactions, IC serotonin a) decreases with the total time focal animals are immobile, and b) positively correlates with the frequency of anogenital investigation (Hall etal. 2011). In contrast, during opposite sex interactions, the level of IC serotonin in focal males decreases with the amount of rejection-like behaviors a male receives from female partners (Fig. 3c) (Keesom and Hurley 2016). These behavioral results are particularly intriguing as they demonstrate that the fluctuation of serotonin within the IC is not only sensitive to external context, but is also an indicator of the valence of event-based changes within individual social interactions.
Fig. 3.
Serotonin in the IC parallels the valence of social interactions for males interacting with females. (A) Females squeak at low levels in the initial phase of interactions that proceed to mounting (light bars), but at high levels in the initial phase of interactions that do not proceed to mounting (gray bars). (B) Serotonin increases in the IC of males following the presentation of novel female partners (“interaction”) as opposed to no partner (“control”). (C) The amplitude of increases in serotonin correlate inversely with the number of female squeaks. Figures adapted from Finton etal. 2017, Anim Behav 126:163–175 (A) and Keesom and Hurley 2016, J Neurophysiol 115:1786–1796 (B, C).
In summary, the predominant characteristic of serotonin release in the IC is its dependence on contextual state. The external contexts that evoke serotonin release are not only varied, but also specific to a small fraction of the circumstances during which serotonergic neurons within DRN are engaged. Specificity in serotonergic signaling emerges in part through inputs from functionally distinct afferent nuclei (Hale and Lowry 2011; Challis and Berton 2015). Of particular interest to context-specific serotonin release in IC are DRN afferents within the social behavior network (SBN).
The social behavior network: an afferent effector of the dorsal raphe?
The SBN, which was first described in mammals (Newman 1999) and later extended to teleost fishes, birds (Goodson 2005), and reptiles (Crews 2003), comprises eight discrete nuclei: the medial extended amygdala (medial amygdala, MeA, and bed nucleus of the stria terminalis, BNST), preoptic area (POA), lateral septum (LS), ventromedial hypothalamus (VMH), anterior hypothalamus (AH), paraventricular hypothalamus (PVN) (Goodson and Kingsbury 2013), the periaqueductal gray (PAG) and ventral tegmental area (VTA). Together, these regions form the core neural architecture for vertebrate social behavior. In mammals, the SBN works in tandem with the mesolimbic reward system in a combined social decision making network (SDMN) which has been proposed to produce and reinforce appropriate social decisions (O’Connell and Hofmann 2011) (but see (Goodson and Kingsbury 2013) for limitations of the SDMN as a pan-vertebrate model). The topographic distribution of individual nuclei or “nodes” across the basal forebrain (LS, MeA, BNST), hypothalamus (VMH, AH, PVN), and midbrain (PAG, VTA) allows the SBN to receive information from functionally and anatomically segregated regions such as memory (Risold and Swanson 1997), endocrine (Tsigos and Chrousos 2002), and sensory systems (Thompson 2005). Reciprocal connections within the SBN allow information from disparate systems to be integrated and processed by each distinct node and the network as a whole. Unsurprisingly, as the SBN is characterized by functional and anatomical heterogeneity, it is the coordinated pattern of activity or “functional connectivity” (Hoke etal. 2005) across the network that establishes the neural contexts (McIntosh 2004) indicative of a particular salient state or behavioral response rather than the activity of a single node (Goodson and Kabelik 2009).
It should not be implied, however, that functional specialization does not exist within SBN. Individual nodes are differentially engaged in a task-dependent manner. For example, the PVN is weighted to modulate neuroendocrine axes (Tsigos and Chrousos 2002), while the BNST is weighted toward valence assessment (Goodson and Wang 2006; Lebow and Chen 2016). Additionally, within discrete nodes, functional specialization is observed at the level of individual cell types: subpopulations of oxytocin and vasopressin-producing neurons in the PVN are generally considered to underlie anxiolytic and anxiogenic processes, respectively, (Kelly and Goodson 2014b). Importantly, functional specialization within different nodes of the SBN mirror functional specialization within the DRN. For example, serotonin increases in IC during restriction stress and direct social contact; these relatively disparate contexts also engage populations of AVP-producing neurons within the PVN and BNST respectively (Ho etal. 2010; Zavala etal. 2011). The functional parallels and anatomical connectivity between subpopulations of AVP neurons and the DRN suggest that the former could gate information on valence/social context to the latter.
While species differences and individual differences exist, AVP-producing neurons are found primarily within five mammalian brain regions: the medial amygdala and BNST in the extended amygdala, and the suprachiasmatic, supraoptic, and paraventricular nucleus (PVN) in the hypothalamus (De Vries and Buijs 1983; Goodson and Bass 2002; De Vries and Panzica 2006; Rood and De Vries 2011; Kelly and Goodson 2014a). AVP exerts modulatory influence via direct synaptic contact or volumetric release from these regions (Albers 2015), and underlies a variety of social behaviors including olfactory processing and species recognition, aggression, affiliation/sociality, pair bonding, and vocal-acoustic production. The diverse functions that AVP influences suggest that it could be a key facilitator of intra-SBN neural context. Likewise, AVP output may signal salient events to extra-SBN nuclei such as DRN. In particular, AVP populations in the PVN and BNST meet several assumptions for a candidate neuromodulator to gate serotonin release into IC. First, AVP immunoreactive (-ir) projections from PVN and BNST are found within DRN (Rood and De Vries 2011; Rood etal. 2013; Pollak Dorocic etal. 2014). Second, these two AVP populations each respond to contexts that trigger serotonin release within the IC.
Within the PVN of rats, restriction paradigms similar to those during which serotonin increases in the IC trigger an up-regulation of AVP mRNA (Bartanusz etal. 1994), and an increased Fos response in AVP-ir neurons (Zavala etal. 2011). Forced swim tests also increase levels of Fos within the PVN (Cullinan etal. 1996) and DRN (Roche etal. 2003), although it is unknown whether serotonin increases in the IC during this paradigm. Functional overlap between the AVP and serotonergic systems is also observed during social defeat: serotonergic neurons in the mouse DRN have an increased Fos response following social defeat (Challis etal. 2013), whereas subordinate male mice have a higher percentage of AVP-ir neurons co-labeled with Fos-ir in the PVN than do their dominant partners after a social interaction (Ho etal. 2010). In addition to these functional similarities, there are direct projections from AVP-ir neurons within PVN to serotonergic neurons in DRN (Pollak Dorocic etal. 2014).
Similar to DRN, BNST serves a role within the circuitry that encodes positive and negative valence (Lebow and Chen 2016; Namburi etal. 2016). The hypothesis that BNST encodes valence sensitivity through functional specialization of cell types was derived from an elegant suite of comparative studies in estrildid finches (family: Estrildidae). Goodson etal. (2005) demonstrated that violet-eared waxbills (Uraeginthus granatina), a territorial estrildid, have a greater Fos response in the BNST than did highly social zebra finches when each was individually engaged with a same-sex conspecific. However, within a subset of AVP-ir neurons in BNST, the opposite is true: gregarious zebra finches have a higher percentage of AVP-Fos-ir co-labeled neurons in response to a same-sex conspecific than do violet-eared waxbills. Importantly, violet-eared waxbills have an increased Fos response in BNST AVP neurons in response to their pair bond partner, suggesting that these neurons code positive social valence (Goodson and Wang 2006). Homologous neurons in mice show a similar response profile: AVP-ir neurons show a large Fos response to copulation, and a smaller yet significant Fos response to male-male chemoinvestigation. AVP producing neurons in the BNST project to intra-SBN targets including the LS and PAG (De Vries and Buijs 1983); BNST AVP neurons are steroid-sensitive, and represent a prominent and evolutionarily conserved sexual dimorphism in the vertebrate brain (De Vries and Panzica 2006; Rood etal. 2013). As in most vertebrates, male mice have more AVP-ir neurons in the BNST than females. Castration not only eliminates AVP-ir neurons in BNST, but also reduces AVP-ir fibers within DRN of male mice, suggesting that BNST is a major source of AVP to DRN (Rood etal. 2013) [but see (Pollak Dorocic etal. 2014)].
DRN afferents affect the production of sensory-driven behavior (Challis etal. 2013; Challis etal. 2014). This can be achieved by modulating the activity of serotonergic neurons either through direct synaptic contact or through non-serotonergic DRN interneurons (Pollak Dorocic etal. 2014; Rood and Beck 2014). Bathing the DRN with AVP indirectly increases the firing rate of serotonergic neurons by activating the vasopressin 1a receptor (V1aR) on excitatory interneurons in vitro (Rood and Beck 2014). Single-cell transcriptomics have revealed that serotonergic neurons within the lateral wings, a sub-region of DRN that projects to the IC (Muzerelle etal. 2016), express the avpr1a gene (Spaethling etal. 2014). In a more systematic approach, two independent studies have employed viral tract tracing techniques to provide an exhaustive list of monosynaptic inputs into the DRN (Pollak Dorocic etal. 2014; Weissbourd etal. 2014). These studies show that AVP neurons target both serotonergic and γ-aminobutyric acid (i.e., GABAergic) neurons within the DRN. Variations in the neurochemical targets of monosynaptic inputs into the DRN are based on the nucleus of origin. For example, AVP-ir neurons within the PVN provide monosynaptic input to serotonergic neurons (Pollak Dorocic etal. 2014), whereas the BNST tends to project more heavily onto DRN GABAergic neurons (though the chemical identity of these projections is unknown) (Weissbourd etal. 2014). Taken together, there is evidence for three potential routes through which AVP neurons could influence the firing of serotonergic DRN neurons: directly, or through glutamatergic or GABAergic intermediaries. These pathways provide an anatomical substrate where functionally distinct types of information, communicated via populations of AVP neurons in the PVN and BNST, could recruit serotonergic neurons in a context-dependent manner.
Functional effects of DRN-IC pathway
The release of serotonin within the IC shapes ascending auditory information. The IC has a rich infrastructure for mediating signals from the DRN. It receives dense serotonergic projections arising mostly from the DRN, and to a lesser extent from the median raphe nucleus and other raphe nuclei (Klepper and Herbert 1991). However, the functional topography of these projections remains unknown [but see: (Muzerelle etal. 2016)]. Serotonergic fibers are seen not only in the mammalian IC, but also in homologous auditory midbrain regions including the anamniote torus semicircularis and avian dorsal lateral mesencephalic nucleus (Cuadrado etal. 1992; Endepols etal. 2000; Matragrano etal. 2012; Matragrano etal. 2013). Once released, serotonin binds to a wide array of different types of serotonin receptors that are expressed by IC neurons themselves; members of five of the seven major families of serotonin receptor have been reported in the IC (reviewed in Hurley and Sullivan 2012). These different receptor types act through divergent intracellular pathways, so that the effect of serotonin release on a given neuron depends on the types of receptors that it expresses, as well as the effects of serotonin on the microcircuitry in which it is embedded (Hurley and Sullivan 2012).
In general, serotonin and its receptors have strong effects on the responses of single IC neurons and neuron populations to acoustic stimuli. Serotonin and its receptors often increase or decrease the amplitudes of responses to simple stimuli like tones, and change the timing of spike trains (Hurley 2006, 2007; Hurley etal. 2008). The integrated effects of multiple receptor types together may shape these aspects of neural responses (Baldan Ramsey etal. 2010). The prevalence of serotonergic effects on responses to simple acoustic stimuli is paralleled by its effects on the responses of single neurons and neuron populations to playback of species-specific vocalizations. In Mexican free-tailed bats (Tadarida brasiliensis), serotonin predominantly increases the selectivity of single IC neurons for an array of social vocalizations, by causing neurons to respond to fewer vocalization types (Hurley and Pollak 2005). This causes the representation of a given call to be more disparate among pairs of neurons on average. In female lab mice, the systemic manipulation of serotonin has effects on immediate early gene activation that depend on both the external context and on estrous phase (Hanson and Hurley 2016). Pharmacologically increasing serotonin release suppresses Fos activity in a non-socially relevant context but not in a socially relevant one. At the same time, elevated serotonin increases Fos activation during proestrus or estrus, but decreases Fos activation during diestrus, while pharmacologically depleting serotonin has the opposite effects. Together, these findings strongly suggest that not only does serotonin shape the responsiveness and selectivity of IC neurons for social vocalizations, but also that its effects are sensitive to contextual state.
Model of contextual feedback to the auditory midbrain
The sections above broadly outline a pathway that could potentially feed back information on the salience of auditory stimuli into the auditory system via the dorsal raphe nucleus. Such a pathway would be capable of importing integrated information from non-auditory sensory channels, as well as “interpreted” representations of external events, such as valence. Although there is strong evidence for each of the individual segments of this pathway, it is not clear which types of behavioral functions it could serve.
In general, such a feedback pathway could modify responses to auditory stimuli based on internal state and salient external circumstances (i.e., contextual state). To illustrate this idea, we will consider a behavioral perception task in lab mice that requires responding to acoustic information in a sex-specific and valence-dependent way: interpreting vocal signals during opposite-sex interaction. Similar to heterosexual interactions in other rodent species, these interactions in lab mice have distinct phases (Pierce etal. 1989). The phases include an initial investigative phase, and a later consummatory phase during which males mount females (Fig. 3a). Whether an interaction proceeds to mounting or not corresponds to female behavior. High amounts of rejection behavior during the initial investigative phase, including the production of human-audible calls (“squeaks”), corresponds to males subsequently reducing courtship behavior (Fig. 3a) (Finton etal. 2017). In contrast, when females show low levels of squeaking and other rejection behavior during the investigative phase, interactions are more likely to proceed to subsequent mounting. When this happens, female squeaks are produced in bursts around mounting events, and overlap in time with a type of male ultrasonic signal with a ∼50 kHz harmonic that is also associated with mounting (Hanson and Hurley 2012). From a male’s perspective, female-produced squeaks may therefore have very different significance in the investigative and consummatory phases of an interaction.
Within the auditory midbrain, serotonin parallels these events, in terms of both its timecourse and valence-dependence (Keesom and Hurley 2016). Voltammetrically measured serotonin increases in the IC of males interacting with females, but not until later time points corresponding to the consummatory phase. Increases in serotonin are also conditionally dependent on female behavior, so that they inversely correlate with the numbers of female squeaks and other rejection behaviors. Thus, serotonin levels would be high in the male IC only under the condition of low levels of initial female rejection, and only at a time when female squeaks are paired with mounting.
Moreover, activating serotonergic pathways in the IC strongly influences responses of IC neurons to playbacks of squeaks. Activating the 5-HT1A receptor, a type which is strongly expressed within the IC (Thompson etal. 1994; Peruzzi and Dut 2004; Smith etal. 2014), greatly decreases the responses of IC neurons to squeaks (Hurley and Nigam 2014). 5-HT1A activation also consolidates squeak-evoked action potentials in time, so that information on the temporally varying acoustic structure of squeaks is also reduced. Combined with the conditional elevation of serotonin in the IC, this means that the representation of female squeaks in the ascending auditory system of males is thus likely to vary with levels of female rejection.
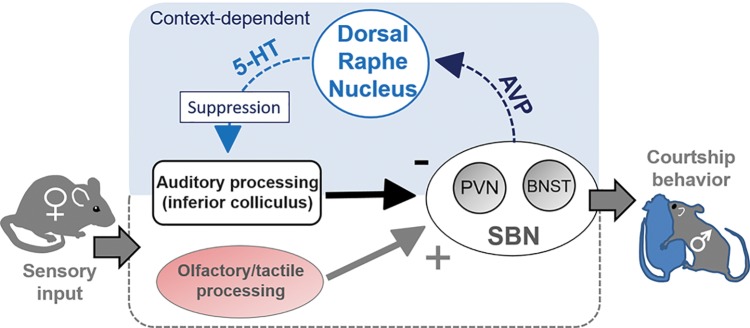
The model of functional circuitry in Fig. 4 represents how all of these events could be tied together. In this model, sensory information regarding female behavior is integrated by at least the level of the social behavior network, and conveyed to the dorsal raphe nucleus by specific neuron populations, such as AVP-expressing neurons in the BNST or PVN. The resulting patterns of serotonergic elevation in sensory regions like the IC therefore depend on a range of contextual factors encoded by these higher regions. As serotonin influences the representation of acoustic stimuli, ascending information on social signals like squeaks is altered. In the model presented in Fig. 4, auditory and nonauditory sensory information plays a crucial role. Cues associated with female presence and acceptance such as tactile or olfactory information play a facilitatory role that ultimately engages serotonin release and corresponds to escalating male courtship and copulatory behaviors. On the other hand, squeaks and other sensory information conveying female rejection play an inhibitory role, decreasing activation of the serotonergic pathway as well as male behaviors.
Fig. 4.
Model of context-dependent feedback from the SBN to the inferior colliculus, through the dorsal raphe nucleus. AVP-positive neurons in the PVN and BNST respond to threatening or social events, and subsequently influence firing rates of neurons in the dorsal raphe nucleus, which release serotonin in sensory regions like the IC. Modulation of firing patterns of IC neurons by serotonin could in turn alter ascending sensory information.
A range of studies supports a generalized model of contextual feedback to sensory systems via the dorsal raphe nucleus. Perhaps the most suggestive piece of evidence in this regard is the observation that neuromodulatory signals within sensory regions reflect not simply the presence of a social partner, but the valence of a specific interaction as it develops (Keesom and Hurley 2016). However, many of the details of the model must still be directly tested to establish how these pathways are engaged during specific behavioral contexts, and whether they are capable of influencing social behavior. It is also important to note that the model is a greatly simplified representation of the intersection among widely projecting systems, which could provide the opportunity for additional layers of feedback. For example, similar to the IC, serotonin release in the medial preoptic area of males occurs during consummatory phases of sexual interaction, and depends on the sexual availability of females (Fumero etal. 1994; Mas etal. 1995; Rubio-Casillas etal. 2015). Broadly projecting neuromodulators such as serotonin from the DRN could thus increase functional connectivity between multiple anatomically and functionally distinct regions within the pathway. Second, there are many projections from nuclei within the SBN to the DRN (Pollak Dorocic etal. 2014; Weissbourd etal. 2014). Multiple projections could therefore act synergistically with populations of AVP neurons to gate information on stressors and social valence. Finally, neurochemical systems other that the ones depicted in Fig. 4, such as noradrenergic or cholinergic pathways, are also engaged by salient behavioral events (Stark and Scheich 1997; Phillips-Farfán and Fernández-Guasti 2009; Metherate 2011; Devilbiss etal. 2012). These additional modulatory systems could also potentially interact with the neural pathways of Fig. 4 at many different levels.
This model can be useful in several different ways. Experimentally, it can support predictions on the coordination of ascending sensory and descending modulatory signals, as well as on the modulation of diverse types of signals that are associated with valence in different ways. This may be relevant for the many signaling systems for which particular signal structures correspond to different circumstances. For example, different vocal signals may be used to court members of the opposite sex versus to signal alarm or distress (Lupanova and Egorova 2015; Egnor and Seagraves 2016). In a system like this, our model might predict the differential regulation of auditory responses to structurally distinct courtship and alarm calls by modulators like serotonin. Thematically, our model suggests that the process of representing external events such as “valence” recruits many neural systems in addition to those that have typically been implicated. In this view, representations like valence may be more akin to transient brain-wide states (i.e., neural contexts (McIntosh 2004)) requiring coordination among many systems. The end result would be to produce a process that is geared, from sensory input to motor output, toward responding appropriately to a dynamic external world.
Acknowledgments
The authors wish to thank two anonymous reviewers whose comments helped to improve this manuscript.
Funding
This work was supported in part by funding from the National Science Foundation [1456298], the National Institute for Deafness and other Communication Disorders [DC006608], by Oak Ridge Associated Universities, and by the Center for the Integrative Study of Animal Behavior at Indiana University.
References
- Albers HE. 2015. Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol 36:49–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Del Negro C, Balthazart J.. 2002. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res 133:221–35. [DOI] [PubMed] [Google Scholar]
- Baldan Ramsey LC, Sinha S, Hurley LM.. 2010. 5-HT1A and 5-HT1B receptors differentially modulate rate and timing of auditory responses in the mouse inferior colliculus. Eur J Neurosci 32:368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartanusz V, Aubry J-M, Steimer T, Baffi J, Kiss JZ.. 1994. Stressor-specific increase of vasopressin mRNA in paraventricular hypophysiotrophic neurons. Neurosci Lett 170:35–8. [DOI] [PubMed] [Google Scholar]
- Bauer EE, Klug A, Pollak GD.. 2002. Spectral determination of responses to species-specific calls in the dorsal nucleus of the lateral lemniscus. J Neurophysiol 88:1955–67. [DOI] [PubMed] [Google Scholar]
- Bernal XE, Stanley Rand A, Ryan MJ.. 2007. Sexual differences in the behavioral response of túngara frogs, Physalaemus pustulosus, to cues associated with increased predation risk. Ethology 113:755–63. [Google Scholar]
- Bester-Meredith JK, Fancher AP, Mammarella GE.. 2015. Vasopressin proves es-sense-tial: vasopressin and the modulation of sensory processing in mammals. Front Endocrinol (Lausanne) 6:5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL.. 2006. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience 143:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley RK, Bass AH.. 1994. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae). Ethology 96:213–32. [Google Scholar]
- Bunin MA, Wightman RM.. 1998. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci 18:4854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H, Young W. III. 2006. Oxytocin and vasopressin: genetics and behavioral implications Handbook of neurochemistry and molecular neurobiology. Springer; p. 573–607. [Google Scholar]
- Caras ML. 2013. Estrogenic modulation of auditory processing: a vertebrate comparison. Front Neuroendocrinol 34:285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, Burmeister SS.. 2009. Estradiol induces sexual behavior in female tungara frogs. Horm Behav 55:106–12. [DOI] [PubMed] [Google Scholar]
- Challis C, Beck SG, Berton O.. 2014. Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front Behav Neurosci 8:43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Berton O.. 2015. Top-down control of serotonin systems by the prefrontal cortex: a path toward restored socioemotional function in depression. ACS Chem Neurosci 6:1040–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O.. 2013. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci 33:13978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF. 2000. The genomic action potential. Neurobiol Learn Mem 74:185–216. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Mohr RA, Sisneros JA.. 2012. Saccular-specific hair cell addition correlates with reproductive state-dependent changes in the auditory saccular sensitivity of a vocal fish. J Neurosci 32:1366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. 2003. The development of phenotypic plasticity: where biology and psychology meet. Dev Psychobiol 43:1–10. [DOI] [PubMed] [Google Scholar]
- Cuadrado MI, Coveñas R, Tramu G.. 1992. Neuropeptides and monoamines in the torus semicircularis of the carp (Cyprinus carpio). Brain Res Bull 29:529–39. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Helmreich DL, Watson SJ.. 1996. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol 368:88–99. [DOI] [PubMed] [Google Scholar]
- Dass SAH, Vyas A.. 2014. Copulation or sensory cues from the female augment Fos expression in arginine vasopressin neurons of the posterodorsal medial amygdala of male rats. Front Zool 11:42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries G, Buijs R.. 1983. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res 273:307–17. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC.. 2006. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience 138:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD, Berridge CW, Valentino R.. 2012. Corticotropin-releasing factor acting at the locus coeruleus disrupts thalamic and cortical sensory-evoked responses. Neuropsychopharmacology 37:2020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Chamero P, Pru JK, Chien MS, Ibarra-Soria X, Spencer KR, Logan DW, Matsunami H, Peluso JJ, Stowers L.. 2015. Cyclic regulation of sensory perception by a female hormone alters behavior. Cell 161:1334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egnor SR, Seagraves KM.. 2016. The contribution of ultrasonic vocalizations to mouse courtship. Curr Opin Neurobiol 38:1–5. [DOI] [PubMed] [Google Scholar]
- Endepols H, Walkowiak W, Luksch H.. 2000. Chemoarchitecture of the anuran auditory midbrain. Brain Res Rev 33:179–98. [DOI] [PubMed] [Google Scholar]
- Finton CJ, Keesom SM, Hood KE, Hurley LM.. 2017. What’s in a squeak? Female vocal signals predict the sexual behaviour of male house mice during courtship. Anim Behav 126:163–75. [Google Scholar]
- Forlano PM, Bass AH.. 2011. Neural and hormonal mechanisms of reproductive-related arousal in fishes. Horm Behav 59:616–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Sisneros JA, Rohmann KN, Bass AH.. 2015. Neuroendocrine control of seasonal plasticity in the auditory and vocal systems of fish. Front Neuroendocrinol 37:129–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumero B, Fernandez-Vera JR, González-Mora JL, Mas M.. 1994. Changes in monoamine turnover in forebrain areas associated with masculine sexual behavior: a microdialysis study. Brain Res 662:233–9. [DOI] [PubMed] [Google Scholar]
- Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav 48:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH.. 2002. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol 448:298–322. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD.. 2005. Neuro-evolutionary patterning of sociality. Proc R Soc Lond B Biol Sci 272:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D.. 2009. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front Neuroendocrinol 30:429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA.. 2013. What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav 64:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y.. 2006. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci U S A 103:17013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley JM, Hazlett EG, Wenstrup JJ.. 2013. Coding the meaning of sounds: contextual modulation of auditory responses in the basolateral amygdala. J Neurosci 33:17538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, Stamper CE, Shekhar A, Lowry CA.. 2008. Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience 157:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Lowry CA.. 2011. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacol (Berl) 213:243–64. [DOI] [PubMed] [Google Scholar]
- Hall IC, Rebec GV, Hurley LM.. 2010. Serotonin in the inferior colliculus fluctuates with behavioral state and environmental stimuli. J Exp Biol 213:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Sell GL, Chester EM, Hurley LM.. 2012. Stress-evoked increases in serotonin in the auditory midbrain do not directly result from elevations in serum corticosterone. Behav Brain Res 226:41–9. [DOI] [PubMed] [Google Scholar]
- Hall IC, Sell GL, Hurley LM.. 2011. Social regulation of serotonin in the auditory midbrain. Behav Neurosci 125:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM. 2016. Serotonin, estrus, and social context influence c-Fos immunoreactivity in the inferior colliculus. Behav Neurosci 130:600–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM.. 2012. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One 7:e40782.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM.. 2014. Context-dependent fluctuation of serotonin in the auditory midbrain: the influence of sex, reproductive state and experience. J Exp Biol 217:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JM, Murray JH, Demas GE, Goodson JL.. 2010. Vasopressin cell groups exhibit strongly divergent responses to copulation and male-male interactions in mice. Horm Behav 58:368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W.. 2005. Social cues shift functional connectivity in the hypothalamus. Proc Natl Acad Sci U S A 102:10712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. 2006. Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J Neurophysiol 96:2177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. 2007. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res 1181:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Bohorquez A, Tracy J.. 2008. The serotonin 1B receptor modulates frequency response curves and spectral integration in the inferior colliculus by reducing GABAergic inhibition. J Neurophysiol 100:1656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD.. 2004. A matter of focus: monoaminergic modulation of stimulus coding within mammalian sensory networks. Curr Opin Neurobiol 14:488–95. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Nigham S.. 2014. The 5-HT1A receptor changes the temporal structure of responses to social vocalizations in the inferior colliculus. 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience.
- Hurley LM, Pollak GD.. 2005. Serotonin selectively modulates responses to species-specific vocalizations in the inferior colliculus. J Comp Physiol A 191:535–46. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Sullivan MR.. 2012. From behavioral context to receptors: serotonergic modulatory pathways in the IC. Front Neur Circ 6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM.. 2001. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed bat, Tadarida brasiliensis. J Comp Neurol 435:78–88. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC.. 1992. Structure and function of the brain serotonin system. Physiol Rev 72:165–229. [DOI] [PubMed] [Google Scholar]
- Keesom SM, Hurley LM.. 2016. Socially induced serotonergic fluctuations in the male auditory midbrain correlate with female behavior during courtship. J Neurophysiol 115:1786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL.. 2014a. Personality is tightly coupled to vasopressin-oxytocin neuron activity in a gregarious finch. Front Behav Neurosci 8:55.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL.. 2014b. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol 35:512–29. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL.. 2015. Functional interactions of dopamine cell groups reflect personality, sex, and social context in highly social finches. Behav Brain Res 280:101–12. [DOI] [PubMed] [Google Scholar]
- Klepper A, Herbert H.. 1991. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res 557:190–201. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD.. 2002. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol 88:1941–54. [DOI] [PubMed] [Google Scholar]
- Lebow MA, Chen A.. 2016. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatr 21:450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-B, Lee HS, Waterhouse BD.. 2008. The collateral projection from the dorsal raphe nucleus to whisker-related, trigeminal sensory and facial motor systems in the rat. Brain Res 1214:11–22. [DOI] [PubMed] [Google Scholar]
- Lupanova AS, Egorova MA.. 2015. [Vocalizations of sex partners in the house mouse (Mus musculus)]. Zh Evol Biokhim Fiziol 51:283–9. [PubMed] [Google Scholar]
- Lynch KS, Ball GF.. 2008. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain Behav Evol 72:207–14. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W.. 2008. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav Evol 71:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL. 2013. The incentive salience of courtship vocalizations: hormone-mediated ‘wanting’ in the auditory system. Hear Res 305:19–30. [DOI] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT.. 2006. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci 23:1523–9. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL.. 2008. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol 511:173–86. [DOI] [PubMed] [Google Scholar]
- Maney DL, Pinaud R.. 2011. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol 32:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC.. 2015. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas M, Fumero B, González-Mora JL.. 1995. Voltammetric and microdialysis monitoring of brain monoamine neurotransmitter release during sociosexual interactions. Behav Brain Res 71:69–79. [DOI] [PubMed] [Google Scholar]
- Matragrano LL, LeBlanc MM, Chitrapu A, Blanton ZE, Maney DL.. 2013. Testosterone alters genomic responses to song and monoaminergic innervation of auditory areas in a seasonally breeding songbird. Dev Neurobiol 73:455–68. [DOI] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Beaulieu M, Sockman KW, Maney DL.. 2012. Estradiol-dependent modulation of serotonergic markers in auditory areas of a seasonally breeding songbird. Behav Neurosci 126:110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. 2004. Contexts and catalysts. Neuroinformatics 2:175–81. [DOI] [PubMed] [Google Scholar]
- Metherate R. 2011. Functional connectivity and cholinergic modulation in auditory cortex. Neurosci Biobehav Rev 35:2058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, Froemke RC.. 2016. A distributed network for social cognition enriched for oxytocin receptors. J Neurosci 36:2517–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P.. 2016. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct Funct 221:535–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namburi P, Al-Hasani R, Calhoon GG, Bruchas MR, Tye KM.. 2016. Architectural representation of valence in the limbic system. Neuropsychopharmacology 41:1697–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevue AA, Elde CJ, Perkel DJ, Portfors CV.. 2015. Dopaminergic input to the inferior colliculus in mice. Front Neuroanat 9:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. 1999. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 877:242–57. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA.. 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 519:3599–639. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Dut A.. 2004. GABA, serotonin and serotonin receptors in the rat inferior colliculus. Brain Res 998:247–50. [DOI] [PubMed] [Google Scholar]
- Petersen CL, Timothy M, Kim DS, Bhandiwad AA, Mohr RA, Sisneros JA, Forlano PM.. 2013. Exposure to advertisement calls of reproductive competitors activates vocal-acoustic and catecholaminergic neurons in the plainfin midshipman fish, Porichthys notatus. PLoS One 8:e70474.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Farfán BV, Fernández-Guasti A.. 2009. Endocrine, neural and pharmacological aspects of sexual satiety in male rats. Neurosci Biobehav Rev 33:442–55. [DOI] [PubMed] [Google Scholar]
- Pierce JD, Sawrey DK, Dewsbury DA.. 1989. A comparative study of rodent ultrasonic vocalizations during copulation. Behav Neural Biol 51:211–21. [DOI] [PubMed] [Google Scholar]
- Pollak Dorocic I, Furth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlen M, Meletis K.. 2014. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 83:663–78. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA.. 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci 11:1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold P, Swanson L.. 1997. Connections of the rat lateral septal complex. Brain Res Rev 24:115–95. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ.. 2003. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci 23:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Beck SG.. 2014. Vasopressin indirectly excites dorsal raphe serotonin neurons through activation of the vasopressin1A receptor. Neuroscience 260:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ.. 2011. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol 519:2434–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, De Vries GJ.. 2013. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol 521:2321–58. [DOI] [PubMed] [Google Scholar]
- Rubio-Casillas A, Rodríguez-Quintero C, Rodríguez-Manzo G, Fernández-Guasti A.. 2015. Unraveling the modulatory actions of serotonin on male rat sexual responses. Neurosci Biobehav Rev 55:234–46. [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Hu B.. 2002. Extracortical descending projections to the rat inferior colliculus. Neuroscience 115:243–50. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Bass AH.. 2003. Seasonal plasticity of peripheral auditory frequency sensitivity. J Neurosci 23:1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH.. 2004. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science 305:404–7. [DOI] [PubMed] [Google Scholar]
- Smith AR, Kwon JH, Navarro M, Hurley LM.. 2014. Acoustic trauma triggers upregulation of serotonin receptor genes. Hear Res 315:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH.. 2017. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct 222:981–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Davis RJ, Gehlert DR, Nomikos GG.. 2006. Exposure to predator odor stress increases efflux of frontal cortex acetylcholine and monoamines in mice: comparisons with immobilization stress and reversal by chlordiazepoxide. Brain Res 1114:24–30. [DOI] [PubMed] [Google Scholar]
- Spaethling JM, Piel D, Dueck H, Buckley PT, Morris JF, Fisher SA, Lee J, Sul JY, Kim J, Bartfai T, et al. 2014. Serotonergic neuron regulation informed by invivo single-cell transcriptomics. FASEB J 28:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H, Scheich H.. 1997. Dopaminergic and serotonergic neurotransmission systems are differentially involved in auditory cortex learning: a long-term microdialysis study of metabolites. J Neurochem 68:691–7. [DOI] [PubMed] [Google Scholar]
- Stoop R, Hegoburu C, van den Burg E.. 2015. New opportunities in vasopressin and oxytocin research: a perspective from the amygdala. Annu Rev Neurosci 38:369–88. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI.. 2008. Patterns of fos activation in rat raphe nuclei during feeding behavior. Brain Res 1200:10–8. [DOI] [PubMed] [Google Scholar]
- Thompson AM. 2005. Descending connections of the auditory midbrain The inferior colliculus. Springer; p. 182–99. [Google Scholar]
- Thompson GC, Thompson AM, Garrett KM, Britton BH.. 1994. Serotonin and serotonin receptors in the central auditory system. Otolaryngol—Head Neck Surg 110:93–102. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP.. 2002. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53:865–71. [DOI] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L.. 2014. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83:645–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Schreiner CE.. 2005. The central auditory system: a functional analysis The inferior colliculus. Springer; p. 1–68. [Google Scholar]
- Xie R, Meitzen J, Pollak GD.. 2005. Differing roles of inhibition in hierarchical processing of species-specific calls in auditory brainstem nuclei. J Neurophysiol 94:4019–37. [DOI] [PubMed] [Google Scholar]
- Xiong XR, Liang F, Zingg B, Ji XY, Ibrahim LA, Tao HW, Zhang LI.. 2015. Auditory cortex controls sound-driven innate defense behaviour through corticofugal projections to inferior colliculus. Nat Commun 6:7224.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JK, Fernandez AA, Gosselink KL.. 2011. Female responses to acute and repeated restraint stress differ from those in males. Physiol Behav 104:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]