Abstract
Study objectives:
To examine the real-world effectiveness of benzodiazepine receptor agonists (BzRAs) by quantifying response and remission rates in a clinical sample receiving chronic BzRA treatment for insomnia.
Methods:
Participants were outpatients (N = 193; 72% female; 55.2 ± 11.1 year) who had an insomnia diagnosis per medical records, and who were taking a therapeutic dose of BzRA for their insomnia. Endpoints were nocturnal sleep disturbance and Insomnia Severity Index (ISI) scores. A reduction meeting the criterion for the minimally important difference in ISI scores (change ≥ 6) constituted “response”; “remission” was inferred when symptoms fell below the clinical cutoff (ISI < 11).
Results:
Most participants (71%) used BzRAs at least 5 nights per week. Mean ISI scores were significantly lower (t = 22.31; p < .01) while on BzRAs than when untreated, but remained in the clinical range (mean = 11.0; standard deviation = 5.7). Although 76.7% responded to treatment, only 47.7% remitted. The majority (68.9%) of participants had a sleep-onset latency > 30 minutes and/or wake-time after sleep onset > 60 minutes while on BzRAs. After controlling for gender and insomnia severity when untreated, odds of insomnia persistence despite BzRA use were 2 times higher in patients with comorbid medical [odds ratio (OR) = 2.39; 95% confidence interval (CI) = 1.20% to 4.77%; p < .05] and psychiatric disorders (OR = 2.24; 95% CI = 1.21% to 4.13%; p < .05).
Conclusions:
This is the first study to distinguish between response and remission in insomnia patients taking BzRAs. Findings suggest that while many insomnia patients respond to chronic BzRA treatment, most do not remit. Remission rates are particularly low for comorbid insomnia, the most prevalent phenotype of the disorder.
Keywords: Benzodiazepine receptor agonists, insomnia, remission, minimally important difference, comorbidity.
Statement of Significance
Use of benzodiazepine receptor agonists (BzRAs) in insomnia treatment is widespread. However, as their evidence base is composed entirely of efficacy trials, the critical question of whether these drugs are effective in clinical settings is unclear. Efficacy trials focus on treatment response, as indexed by reductions in the sample mean on specific sleep disturbance parameters. By contrast, the proportion of patients who remit from insomnia disorder is seldom assessed. The present study indicates that large proportions of insomnia patients fail to remit despite almost nightly BzRA use, with higher rates in those with medical/psychiatric comorbidities. Incomplete remission may be a risk factor for not just relapse, but sustained risk for other insomnia-related morbidities.
INTRODUCTION
Benzodiazepine receptors agonists (BzRAs) are the most commonly prescribed of the US Food and Drug Administration (FDA) medications indicated for insomnia.1 However, nearly all the empirical support for these agents derives from efficacy trials, with limited research on effectiveness in clinical samples. Efficacy trials suffer from 2 systematic limitations. First, the primary endpoints in the vast majority of efficacy trials are improvements in the sample mean on nocturnal sleep disturbance measures.2 Their focus is on evaluating a minimally important difference (MID) in specific nocturnal symptoms, which is more an index of treatment response than of remission per se. A second shortcoming of most efficacy trials is that they exclude patients suffering from the most prevalent phenotype of the disorder, i.e., insomnia comorbid with medical and psychiatric disorders.3–5 As such, efficacy in a controlled trial may not translate to effectiveness in clinical practice. A recent report6 identified a series of sleep clinic patients with persistent insomnia symptoms despite chronic nightly use of prescription sleep-aids, of which BzRAs were the most commonly used and accounted for two-thirds of all sleep-aids. However, as only nonremitters were included in this study, rates of remission (and nonremission) from insomnia disorder among BzRA users in clinical settings is currently unknown. What is the scope of this problem? Further, it is also unclear which, if any, specific patient characteristics may compromise the effectiveness of BzRA treatment. As always, the more pertinent question is not whether a particular treatment works, but for whom it is an appropriate therapeutic option.
While acute and transient nocturnal sleep disturbances, including increased sleep-onset latency (SOL) and wake-time after initial sleep onset (WASO), are ubiquitous, 6% to 10% of the population meets diagnostic criteria for insomnia disorder.7 An important distinction is that insomnia is a not just a nocturnal disorder but one involving concurrent daytime impairments, with growing evidence of elevated arousal throughout the 24-hour day.8–10 Yet, the endpoints in most BzRA efficacy trials are specific nocturnal sleep disturbance parameters. Even in pharmacological trials evaluating long-term BzRA use, efficacy probes still target sleep onset/maintenance measures. For instance, in one of the longest clinical trials of an FDA-approved BzRA sleep aid,11 efficacy was assessed for 2 consecutive nights at the 1-month and 8-month marks following randomization. However, the endpoints were sleep disturbance on those particular nights, and not insomnia remission at the end of treatment. With respect to the assessment of daytime function, the goal in efficacy trials is not to evaluate improvements in insomnia-related daytime impairment, but to rule out the residual sedative effects of the sleep aid.2 Thus, most BzRA trials assess treatment response in terms of improved nocturnal sleep and residual drug effects, neither of which adequately captures insomnia remission.
In the relatively few BzRA trials which have examined remission using empirically validated outcomes, insomnia remission rates are modest (46% to 55%) relative to placebo (20% to 40%).12,13 An important question therefore arises: are remission rates even lower in clinical settings where most patients present with comorbid disease? Psychiatric/medical disorders are comorbid with insomnia in 70% to 86% of cases,14–16 and have been proposed as an important factor in the effectiveness of prescription sleep-aids.6 The exclusion of patients with medical and psychiatric disorders in efficacy trials has been criticized for this reason since as early as 1989.3–5 In its prescribing information (label) for 2 common BzRAs, the FDA notes that nonremission from insomnia while taking these medications may reflect a comorbid condition warranting assessment.17,18 However, efforts to examine the efficacy of BzRAs in treating insomnia comorbid with another disorder have only begun recently. Efficacy data for BzRAs are currently available for insomnia comorbid with major depressive disorder (MDD),19–21 generalized anxiety disorders (GAD),22–24 rheumatoid arthritis,25,26 menopause,27,28 and chronic back pain.29 There is still considerable disparity between the small number of disorders studied in these trials and those that accompany insomnia in clinical settings.3
Effectiveness studies in clinical samples can complement efficacy trials by addressing some of these limitations and providing essential real-world data on treatment outcomes. However, nearly all BzRA research beyond efficacy trials has focused on epidemiological questions: trends in the prescribing habits of physicians, the prevalence of use in the general population, and the association between chronic use and adverse consequences.30–36 Only 2 prior studies have examined effectiveness.37,38 Neither study distinguished between sleep complaints and insomnia disorder and, as such, response to treatment and remission from the disorder could not be extricated. Vital information on the dose and pattern of prescription sleep aid use was missing from both studies. Additionally, important characteristics such as comorbid psychiatric/medical illness, were unavailable. To address these gaps in the literature, the present study assessed outcomes of BzRA treatment in a clinical sample of insomniacs. Information on dosage and frequency of BzRA use was collected, and only patients who reported therapeutic dosages and “indicated use” for insomnia were retained. As an insomnia disorder diagnosis is symptom based, patient-reported outcomes offer the most valid metric for gauging treatment effectiveness and were hence the primary endpoints in this study. Treatment “response” was determined per empirically validated criteria for the MID in symptomatology,39 and remission was operationalized as a reduction in symptoms to subclinical levels.40
METHODS
Setting and Participants
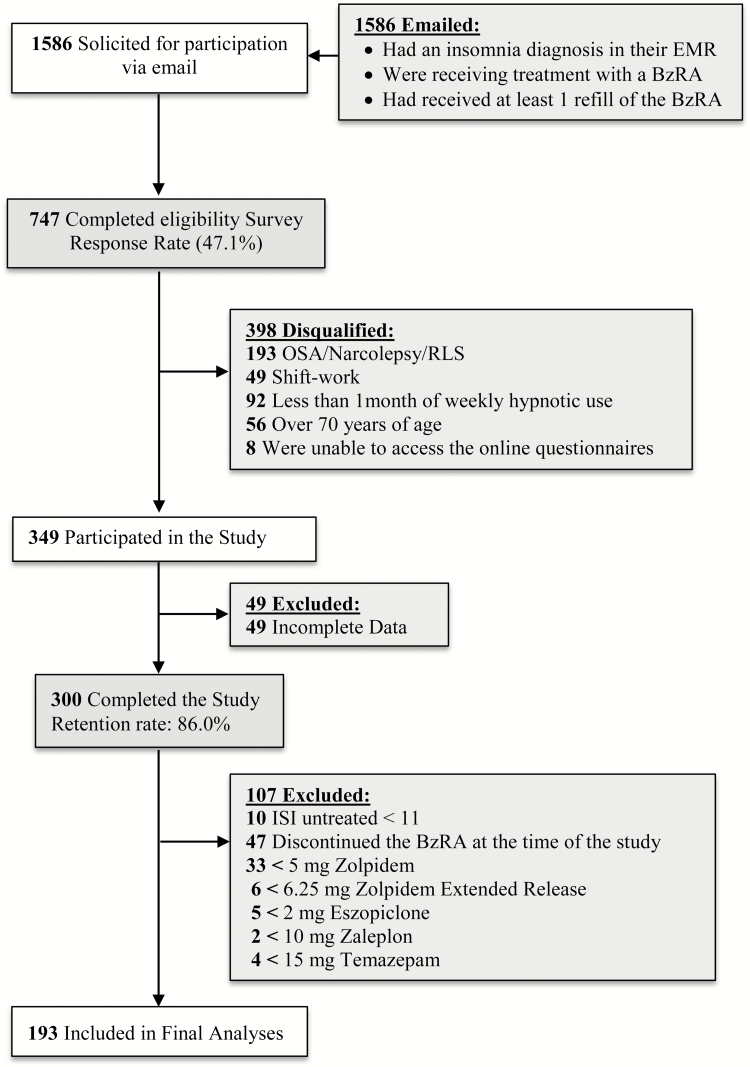
Participants were recruited from a central repository which included electronic medical records of all patient encounters within the Henry Ford Health System. Over 3 million patient encounters are added to this repository each year, and the patient sample reflects the gender, race, and ethnic composition of Southeastern Michigan. Outpatients from this database were invited to participate via email if: (1) they had received an insomnia diagnosis in the past 2 years; (2) were prescribed a BzRA medication for insomnia (see Measures section for a list of eligible medications); and (3) had received at least one refill of this BzRA. This email invitation included the web address (URL) for a screening questionnaire which assessed additional inclusion/exclusion criteria (Figure 1). Inclusion criteria were: age 20 years or more; exclusion criteria were: other sleep disorders, including sleep apnea, restless legs syndrome, narcolepsy; age older than 70 years; night, evening, rotating shift-work, on-call work-schedule (Table 1). This electronic screening questionnaire was administered using Qualtrics, an internet-based software suite developed by Qualtrics Inc. (Provo, UT). Eligible participants were automatically directed to the full survey, which was also completed via Qualtrics. The institutional review board at the Henry Ford Health System approved this protocol. Demographic characteristics of excluded cases (n = 107) did not diverge significantly from the final sample: (age: 53.1 ± 11.2 year; 71.2% female). Levels of medical/psychiatric comorbidities were also comparable (depression: 39.4%; anxiety: 29.8%; hypertension: 46.2%; high cholesterol: 39.4%).
Figure 1.
Flow of participants through the study. EMR = electronic medical record; BzRA = benzodiazepine receptor agonist; OSA = obstructive sleep apnea; RLS = restless leg syndrome.
Table 1.
Distribution of BzRA Use (n = 193).
| Agenta | Trade name | Minimal therapeutic dose | Prevalence n (%) |
|---|---|---|---|
| Zolpidem | Ambien | 5 mg | 138 (71.5) |
| Temazepam | Restoril | 15 mg | 22 (11.4) |
| Eszopiclone | Lunesta | 2 mg | 13 (6.7) |
| Zolpidem ext. release | Ambien CR | 6.25 mg | 12 (6.2) |
| Zolpidem oral spray | Zolpimist | 5 mg | 2 (1.0) |
| Zolpidem sublingual | Intermezzo | 1.75 mg | 2 (1.0) |
| Flurazepam | Dalmane | 15 mg | 1 (0.5) |
| Triazolam | Halcion | 0.125 mg | 2 (1.0) |
| Zaleplon | Sonata | 10 mg | 1 (0.5) |
BzRA = benzodiazepine receptor agonist; mg = milligrams.
aThe following BzRAs were assessed, but not endorsed by any participants: Doral (Quazepam); Edluar (Sublingual Zolpidem); Prosom (Estazolam).
Measures
Insomnia
Eligible participants rated insomnia severity using the Insomnia Severity Index (ISI).41 The ISI is a 7-item self-report questionnaire, which has been validated for detecting the caseness of insomnia disorder, quantifying severity, and assessing treatment response.39,40 Participants completed 2 separate versions of the ISI: one referencing the period when they were untreated, and the other targeting the period when they were on BzRAs for their insomnia. The latter began with the following prompt: “Please rate the severity of your insomnia symptoms while taking [endorsed BzRA]”. By contrast, the ISI for the untreated period included the following prompt: “Please rate the severity of your insomnia symptoms while NOT taking any insomnia medication”. The same strategy was used to assess levels of sleep disturbance, i.e., SOL and WASO.
BzRA Use
Participants reported the specific BzRA they were taking for the insomnia, as well as the dosage and frequency of use. They also listed all other medications they were taking (Supplemental Material).
Response and Remission
The MID cutoff (ISI reduction ≥ 6) to operationalize treatment response was based on prior research on the ISI as an index of therapeutic change. Yang and colleagues39 found that a 6-point reduction in ISI scores was reflective of clinically significant improvements in health-related outcomes, such as fatigue, daytime functioning, and quality of life. With respect to remission, a cutoff of 11 on the ISI shows the highest sensitivity and specificity in detecting caseness of insomnia disorder in clinical samples.40 As such, participants who scored below 11 while taking BzRAs were considered remitted.
Data Analysis
Statistical analyses were conducted using IBM SPSS Statistics, version 23 (Armonk, NY).42 McNemar χ2 tests were conducted to examine whether the proportion of participants with difficulties initiating sleep or maintaining sleep were significantly different on BzRAs compared to when untreated; this strategy accounts for repeated measures and the binary nature of outcomes. Similarly, a paired-samples t test was used to examine whether ISI score was significantly different while taking BzRAs compared to when untreated. Univariate between-group comparisons (e.g., participants with comorbid medical/psychiatric disorders vs. those without comorbidities) were done using independent samples t tests (continuous outcomes) and Pearson χ2 tests of independence (dichotomous outcomes) as appropriate. For more complex between-group models involving multiple covariates and dichotomous outcomes (remitters vs. nonremitters), logistic regression analyses were used. Covariates for logistic regression models were selected per hypotheses and/or if they were related to the outcome variable in univariate analyses at a significance level of p < .20.43
RESULTS
A small proportion of respondents (16.7%) reported using less than the minimal therapeutic dose of the endorsed BzRA (Figure 1). These cases were stricken from analyses. Another 47 participants (15.7%) had discontinued BzRA use at the time of the study. The most commonly cited reasons for ceasing use were side effects (40.4%) and lack of efficacy (27.7%); none of these participants attributed BzRA discontinuation to a resolution of sleep problems. Finally, ISI scores while untreated were lower than the clinical cutoff (<11) for only 10 participants (3.3%), who were also excluded from analyses.1
Sample Characteristics
Demographic characteristics of the final sample appear in Table 2. Participants were predominantly middle-aged (55.2 ± 11.1 year) and “white” (77.2%), though some demographic diversity was observed (African American: 12.4%; more than 1 race: 4.1%; Asian: 2.6%). Women were over-represented (72.0%), as expected per the gender disparity in the prevalence of insomnia.44 Of the overall sample, 11.4% reported no psychiatric or medical comorbidity. About half (50.3%) of the sample presented with psychiatric comorbidities, of which depression (39.4%) and anxiety (31.1%) were the most common. Hypertension was the most prevalent medical comorbidity (48.7%), followed by high cholesterol (42.0%) and chronic pain (39.9%). Overall, 73.6% of the sample presented with some form of medical comorbidity. The proportion of smokers was 10.8%, and mean alcohol use was 1.4 beverages per week. As for concomitant medications (Supplement), lisinopril (14.3%) and simvastatin (10.8%) were the most common. About a third (27.7%) of the sample was on antidepressants, of which sertraline (7.9%) and bupropion (5.4%) were the most widely used. Anxiolytic use was reported by 13.8% of the sample; alprazolam (6.4%) was the most common, followed by lorazepam (3.4%).
Table 2.
Sample Characteristics, Stratified by Insomnia Response/Remission While Taking BzRAs.
| Full sample | Nonresponders | Responders | Nonremitters | Remitters | |
|---|---|---|---|---|---|
| n = 193 | n = 45 | n = 148 | n = 101 | n = 92 | |
| % | % | % | % | % | |
| 100 | 23.3 | 76.7 | 52.3 | 47.7 | |
| Gender (women) | 72.0 | 55.6 | 77.0 | 66.3 | 78.3 |
| Race (white) | 77.2 | 68.9 | 79.7 | 75.2 | 79.3 |
| Smoker (yes) | 10.4 | 6.7 | 11.5 | 9.9 | 10.9 |
| Psyc. comorbidity | 50.3 | 51.1 | 50.0 | 59.4 | 40.2 |
| Med. comorbidity | 73.6 | 86.7 | 69.9 | 81.2 | 65.2 |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Age (years) | 55.2 (11.1) | 56.5 (10.5) | 54.8 (11.3) | 55.2 (10.2) | 55.1 (12.2) |
| Alc. drinks/week | 1.4 (2.2) | 1.2 (1.9) | 1.4 (2.2) | 1.4 (2.2) | 1.4 (2.2) |
| ISI untreated | 21.1 (4.3) | 19.2 (4.7) | 21.7 (4.0) | 21.6 (3.9) | 20.5 (4.7) |
| ISI while on BzRAs | 11.0 (5.7) | 16.6 (5.0) | 9.2 (4.7) | 15.3 (3.9) | 6.2 (2.9) |
| Frequency of BzRA use (days/week) | 5.6 (2.0) | 5.0 (2.2) | 5.8 (1.9) | 5.5 (2.0) | 5.7 (2.9) |
BzRA = benzodiazepine receptor agonist; Cig. = cigarettes; ISI = insomnia severity index; M = mean; Med. = medical; Psyc. = psychiatric; SD = standard deviation.
BzRA Use
Zolpidem was the most frequently used BzRA, endorsed by 71.5% of the sample. Temazepam and eszopiclone were endorsed by 11.4% and 6.7%, respectively, and were the second and third most commonly used BzRAs. Dose distribution of zolpidem was fairly even: 45.7% took the 5 mg dose, 49.3% were on 10 mg. Most eszopiclone users (61.5%) were on the 3 mg dose, whereas the 30 mg dose (40.4%) was the most common among temazepam users. Frequency of BzRA use was high (mean (M) = 5.6 day/weeks; standard deviation (SD) = 2.0), with a median of 7 days per week. The majority (71%) of the sample reported using BzRAs at least 5 days per week. Frequency of use did not vary significantly across BzRAs. Roughly half the sample (51.2%) had tried 2 or more prescription medications for their insomnia in the past.
Nocturnal Sleep Disturbance
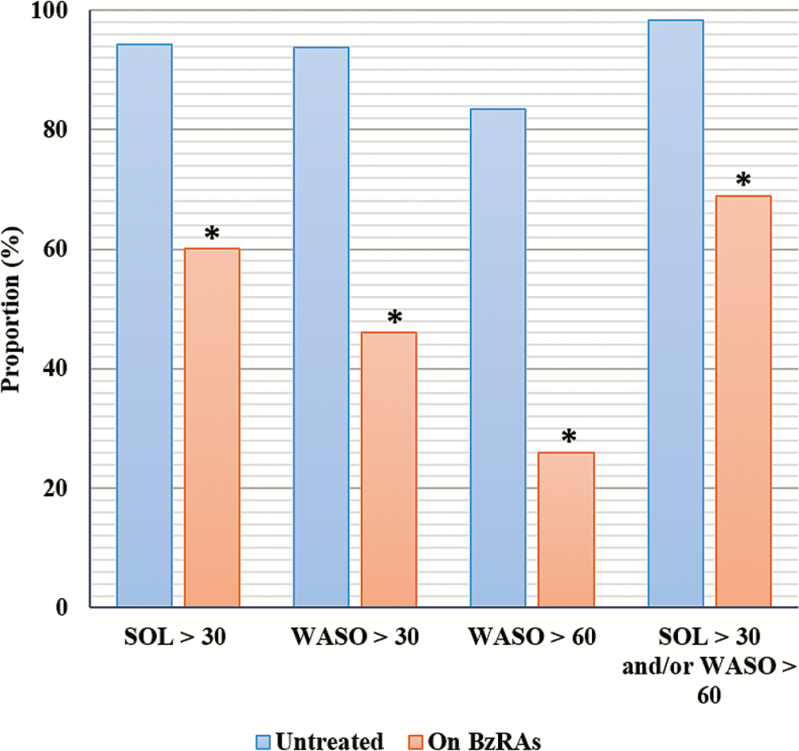
As Figure 2 shows, nearly all participants reported elevated SOL and WASO45 while untreated. Overall proportions of participants endorsing these sleep disturbances while taking BzRAs were significantly lower than while untreated (all p values < .001; Table 3). However, a large majority (68.9%) reported SOL >30 minutes and/or WASO >60 minutes while taking BzRAs.
Figure 2.
Sleep disturbances while on BzRAs. BzRA = benzodiazepine receptor agonist; SOL = sleep onset latency; WASO = wake-time after sleep onset; *all McNemar’s chi-square tests were significant at p < .001.
Table 3.
Sleep Disturbance While on BzRAs.
| Untreated (%) | On BzRAs (%) | McNemar’s χ2 | |
|---|---|---|---|
| SOL > 30 m. | 94.3 | 60.1 | 63.64; p < .001 |
| WASO > 30 m. | 93.8 | 46.1 | 88.48; p < .001 |
| WASO > 60 m. | 83.4 | 25.9 | 109.21; p < .001 |
| SOL > 30 m. and/or WASO > 60 m. | 98.4 | 68.9 | 55.02; p < .001 |
BzRA = benzodiazepine receptor agonist; m = minutes; SOL = sleep onset latency; WASO = wake-time after sleep onset.
ISI Scores While Untreated
Participants reported high ISI scores while untreated (M = 21.1; SD = 4.3; median = 21; Table 4). An independent samples t test comparing men and women on mean ISI scores (men: M = 19.4; SD = 4.0; women: M = 21.7; SD = 4.3) was statistically significant (t = 3.52; p < .05). Between-group comparisons of participants with psychiatric comorbidities (M = 21.9; SD = 4.2) and those without (M = 20.2; SD = 4.3) also yielded a statistically significant difference (t = 2.73; p < .05). Participants with medical comorbidities (M = 21.2; SD = 4.4) and those without (M = 20.7; SD = 4.2) did not differ (t = 0.75; p = .45).
Table 4.
Group Differences in ISI Scores While Untreated.
| ISI scores while untreated | Independent samples t test | |
|---|---|---|
| M ± SD (n) | ||
| Gender | ||
| Men | 19.4 ± 4.0 (54) | 3.52; p < .05 |
| Women | 21.7 ± 4.2 (139) | |
| Racea | ||
| White | 21.2 ± 4.2 (149) | 0.90; p = .37 |
| African American | 20.3 ± 4.9 (24) | |
| Smoker | ||
| Yes | 21.2 ± 4.9 (20) | 0.08; p = .94 |
| No | 21.1 ± 4.3 (173) | |
| Medical comorbidity | ||
| Without | 20.7 ± 4.2 (51) | 0.75; p = .45 |
| With | 21.2 ± 4.4 (142) | |
| Psychiatric comorbidity | ||
| Without | 20.2 ± 4.3 (96) | 2.73; p < .05 |
| With | 21.9 ± 4.2 (97) | |
ISI = insomnia severity index; M = mean; n = sample size; SD = standard deviation.
aResults are presented for the 2 most commonly endorsed categories of the Race variable (“White” and “African American”) as there were two few cases of other race categories for meaningful comparisons.
Responders and Remitters
A paired-samples t test showed that ISI scores while on BzRAs were significantly lower than while untreated (t = 21.31; p < .01), but still remained in the clinically significant range (M = 11.0; SD = 5.7; median = 11). A high percentage (76.7%) of participants showed a therapeutic response to BzRAs. However, only 47.7% of the sample achieved remission (59.5% of responders). To examine whether response and remission rates varied as a function of BzRA dose, the sample was divided into 2 groups (high vs. low dose of the respective BzRA). Dose was unrelated to response (χ2 = .04; p = .83) or remission rates (χ2 = .26; p = .61).
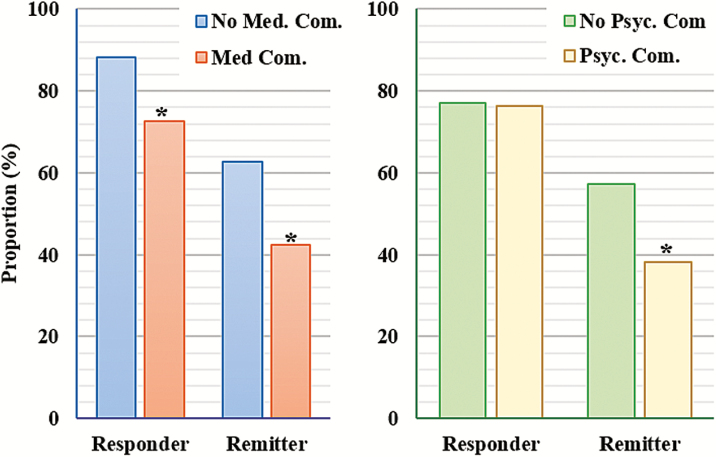
Chi-square tests of independence showed that participants with medical comorbidities had significantly lower response (72.5% vs. 88.2%) and remission rates (42.3% vs. 62.7%) than did those without medical illness (all p values < .05; Figure 3). Participants with comorbid psychopathology had significantly lower remission rates (38.1% vs. 57.1%) than those without, but response rates did not differ between these groups (Table 5). To further explore these findings, we examined response and remission rates, controlling for untreated ISI scores and pertinent covariates (p <.20 in univariate analyses).
Figure 3.
Response and remission rates, stratified by medical/psychiatric comorbidity. Med = medical; Pscy = psychiatric; Com = comorbidity. *p < .05.
Table 5.
Response and Remission Rates Stratified by Medical/Psychiatric Comorbidity.
| Response rate | Pearson’s χ2 | Remission rate | Pearson’s χ2 | |
|---|---|---|---|---|
| % | % | |||
| Med. Com. | 5.17; p < .05 | 6.32; p < .05 | ||
| Without | 88.2 | 62.7 | ||
| With | 72.5 | 42.3 | ||
| Psyc. Com. | 0.17; p = .90 | 7.09; p < .05 | ||
| Without | 77.1 | 57.3 | ||
| With | 76.3 | 38.1 |
Com = comorbidity; Med = medical; Psyc = Psychiatric.
First, we fit a logistic regression model with treatment response as the dependent variable (0 = responder; 1 = nonresponder). Gender, psychiatric comorbidity, medical comorbidity, and ISI scores while untreated served as the independent variables (IVs). A test of the model with all predictors against a constant-only model was statistically significant (χ2 = 22.83; p < .01), indicating that this model reliably distinguished between responders and nonresponders. The Hosmer–Lemeshow test showed that the model fit the data well (χ2 = 6.65; p = .58). Odds of responding were significantly lower among participants with medical comorbidities (Table 6). Stated another way, participants with medical comorbidities were significantly more likely to not respond [odds ratio (OR) = 3.24; p < .05].
Table 6.
Multivariate Models of Treatment Nonresponse and Insomnia Persistence.
| OR (95% CI) nonresponse | Wald’s χ2 | OR (95% CI) insomnia persistence (nonremission) | Wald’s χ2 | |
|---|---|---|---|---|
| Med. Com. | 3.24 (1.22–8.56) | 5.66; p < .05 | 2.39 (1.20–4.77) | 6.09; p < .05 |
| Psyc. Com. | 1.43 (0.69–2.98) | 0.93; p = .33 | 2.24 (1.21–4.13) | 6.61; p < .05 |
| Overall modela: χ2= 22.83; p < .001 | Overall modela: χ2 = 21.30; p < .001 | |||
CI = confidence interval; Com = comorbidity; Med = medical; OR = odds ratio; Psyc = psychiatric.
aBoth logistic regression models controlled for gender and ISI scores while untreated.
We fit a similar logistic regression model with remission status as the outcome (0 = remission; 1 = nonremission, i.e., insomnia persistence); again, the IVs were gender, psychiatric comorbidities, medical comorbidities, and ISI scores while untreated. A test of this model with all predictors against a constant-only model was statistically significant (χ2 = 21.32; p < .01), and fit the data well (Hosmer–Lemeshow test: χ2 = 6.89; p = .55). Psychiatric comorbidity (OR = 2.24; p < .05) and medical comorbidity (OR = 2.39; p < .05) were each associated with significantly higher odds of insomnia persistence.
DISCUSSION
The present study examined whether outpatient insomniacs responded to chronic BzRA treatment with meaningful improvements, and more importantly, if such changes were accompanied by a remission of insomnia disorder. As clinicians rely on patient reports to diagnose insomnia, we focused on patient- reported outcomes; more objective assessment techniques, such as polysomnography, are not indicated for diagnosing insomnia and are rarely utilized in clinical practice.46 Results showed that treatment response was high. For most patients (76%), treatment-related changes in symptoms were above the threshold for a MID (ISI change ≥ 6).39 Proportions of participants reporting sleep disturbance above conventional quantitative cutoffs45,47 were also significantly lower while taking BzRAs. Insomnia remission rate (47.7%), however, was less promising, with over half the sample reporting clinically significant insomnia levels (ISI ≥ 11)40 despite almost nightly BzRA use. Remission rates were even lower in patients with medical comorbidities (42%) and those with comorbid psychopathology (38%). This finding is particularly noteworthy as 88.6% of insomniacs in the present study presented with some psychiatric/medical comorbidity; prior studies suggest a similar prevalence (86%) of comorbid disease in insomniacs.16 Overall, this study points out that BzRAs are not fully effective in large numbers of patients. Prior data on the wide-scale effectiveness of these sleep-aids likely capture treatment response and not remission.37,38
BzRA Treatment for Comorbid Insomnia
Insomnia patients rarely present without some form of medical/psychiatric comorbidity,48 a clinical reality which calls into question the common practice of excluding medical/psychiatric illness from efficacy trials. In the only prior study of patients whose insomnia was resistant to treatment with prescription sleep-aids including BzRAs, levels of comorbid psychopathology were high.6 However, this study lacked a comparator group of treatment responders, rendering it difficult to implicate comorbid psychopathology in treatment- resistance. Another report presented post hoc analyses of BzRA treatment from 5 different efficacy trials, including patients with “primary” insomnia and those with insomnia comorbid with depression, GAD, menopause, and arthritis.49 Outcome effect sizes were the largest for primary insomnia and the smallest for insomnia comorbid with GAD/MDD. However, these analyses were restricted to a single-dose of a single-agent. Our findings support the hypothesis that comorbid conditions undermine the effectiveness of other, commonly prescribed BzRAs. The important question therefore is what drives this effect?
Insomnia severity is an unlikely candidate. Two prior studies4,5 compared insomniacs who participated in efficacy trials with insomniacs who presented to sleep clinics. Though comorbid disease was more common in the latter, the two groups did not differ on the severity of any sleep disturbance parameter. This finding was replicated in our study. Notably, differences in ISI scores while untreated between patients with and without comorbid psychopathology were statistically significant. However, scores in both groups were elevated, and the difference was small and unremarkable by any clinical standards. ISI scores while untreated in insomniacs with and without medical comorbidities were also comparable (Table 4).
Another possibility is that adjunctive treatment of comorbidities is a factor in the effectiveness of BzRAs. As we lacked detailed information on the dosage and use-frequency of nonsleep medications, we were unable to examine the potential sleep/daytime effects of other agents. Interestingly, treatment response to antidepressant medications when combined with a BzRA for insomnia has been tested against monotherapy with antidepressants (i.e., antidepressant + BzRA vs. antidepressant + placebo).19,20 However, there are no studies on the hypnotic effects of BzRAs as a function of adjunctive therapy for a comorbid condition (BzRA + other agent vs. BzRA + placebo). Such studies are needed to examine the effectiveness of BzRAs in the majority of insomnia patients, who are likely receiving treatment for comorbid medical/psychiatric disorders.
Implications for Alternative Treatments
An important research priority in light of the large proportion of nonremitters in the present study is testing the effectiveness of non-BzRA agents in this population. Novel pharmacological agents with alternative mechanisms of action, including low-dose doxepin, a histamine antagonist, and suvorexant, a dual orexin receptor antagonist, have recently received FDA approval for insomnia treatment.50–52 Off-label use of other agents, including sedative antidepressants, for insomnia is also common.53 Overall, at least 11 different classes of medications are currently used in the treatment of insomnia in the United States.54 However, the evidence base of dose-related risks and benefits for non-BzRA agents is considerably limited compared to that for BzRAs.48 With a few exceptions, such as doxepin and ramelteon, Finally, although the availability of a wide array of pharmacological agents can help physicians tailor treatment per individual patient needs, pharmacotherapy in general may not be appropriate for all patients. Half the current sample had tried more than 2 different medications for their insomnia in the past, which suggests that multiple treatment attempts with different pharmacological agents is not uncommon in clinical practice. Continuing pharmacotherapy after multiple medications have proven ineffective is ill-advised.53 Patients for whom a nonpharmacological alternative is more appropriate may benefit from cognitive-behavior therapy for insomnia, as empirical support for this modality is extensive.55,56
Limitations and Future Directions
The primary limitation of this study is that insomnia symptoms “while untreated” were based on retrospective report. Future effectiveness studies can rule out this confound by using prospective designs. Another weakness of this study is that data on the duration of insomnia, an important covariate, were unavailable. Furthermore, as many insomniacs do not seek treatment, there is some risk of selective sampling in the present study.57 Another factor limiting the generalizability of findings is that participants with primary sleep disorders, such as OSA, were excluded. We also note that this report addresses chronic BzRA use, as opposed to intermittent or short-term use. With respect to differences in the proportions of participants reporting sleep disturbance (e.g., SOL > 30, WASO > 60) while taking BzRAs, there is some risk of type 1 error due to multiple inference tests. Although significance tests were robust to family-wise alpha corrections, this adjustment strategy offers only a facile solution to the problem.58 We argue that the interpretation of these data does not rest on “statistical significance.” Instead, the practical value of these findings is in the identified proportions of patients reporting sleep disturbance despite BzRA use.
Effectiveness studies in clinical settings occupy the middle ground between randomized controlled pharmacological trials on one end and pharmacoepidemiological studies on the other. By definition, effectiveness studies lack the methodological precision of efficacy trials. As such, we were unable to address more nuanced outcomes, such as equipotency of different BzRAs or dose-responses curves, primarily due to the disproportionately high prevalence of zolpidem use in the present sample. Notably, this reflects nationwide increases in the prescription rates of nonbenzodiazepine BzRAs for insomnia over the past 2 decades, and the nearly commensurate decrease in traditional benzodiazepine prescriptions during the same period.59 While remaining consistent with epidemiological findings, this study has many additional strengths, including recruitment of actual insomnia patients, use of empirically validated instruments, and limiting BzRA cases to therapeutic dosages and indicated use for insomnia. As such, we avoided common pitfalls in pharmacoepidemiology, such as “confounding by indication” and poor characterization of insomnia.
The distinction between response and remission, while commonly addressed in outcome research for other disorders such as MDD,19,60 has not been examined adequately in insomnia pharmacotherapy. Although BzRAs are the most widely used pharmacological agents in the treatment of insomnia, our findings point to an important limitation: while many patients improve, most do not remit. This is a significant health concern, as insomnia is a well-established risk factor for a number of disorders, including MDD and hypertension.61–64 Do insomniacs who respond to treatment but fail to remit remain at increased risk for these morbidities? This is an important question for future research. There are a number of ongoing investigations on insomnia treatment as a preventive strategy against disorders such as MDD.65,66 The distinction between treatment response and remission may be an important consideration in such prevention efforts.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at Sleep online.
FUNDING
This study was supported by a grant from Merck (#MK 52864) to Christopher L. Drake (PI).
DISCLOSURE STATEMENT
Dr. Pillai has received research support from Merck. Dr. Roth has been a consultant for Merck, Inc., Sanofi-Aventis, Purdue, Cephalon, Transcept, Glaxo Smith-Kline, Jazz, Pfizer, Bayer, Johnson & Johnson. He has received research support from Merck, Cephalon, Transcept. He has served on speakers’ bureaus for Purdue. Dr. Roehrs has been a consultant for Sanofi-Aventis, Sepracor, Elan. He has received research support from Sanofi-Aventis. He has served on speakers’ bureau for Sepcracor. Dr. Moss did not report any conflicts of interests. Dr. Peterson did not report any conflicts of interests. Dr. Drake has received research support from Merck, Teva, Pernix, Intelclinic, Aladdin Dreamers. He has served on speakers’ bureau for Merck, Teva.
ACKNOWLEDGMENTS
This manuscript is dedicated to the memory of Vivek Pillai whose life was tragically cut short just after completion of this work. Vivek was a tremendously dedicated and rising star in the sleep field and made significant contributions including the present work at a very early stage of his career. Our thoughts and prayers are with his friends and family. This study was conducted at the Sleep Disorders and Research Center at Henry Ford Hospital.
REFERENCES
- 1. Ebert B, Wafford KA, Deacon S. Treating insomnia: Current and investigational pharmacological approaches. Pharmacol Ther. 2006; 112(3): 612–629. [DOI] [PubMed] [Google Scholar]
- 2. Walsh JK, Roth T. Pharmacologic treatment of insomnia benzodiazepine receptor agonists. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine (Sixth Edition). Vol 6 6th ed. St. Louis, MO: Elsevier; 2016: 832–841. [Google Scholar]
- 3. Krystal AD. A compendium of placebo-controlled trials of the risks/benefits of pharmacological treatments for insomnia: the empirical basis for U.S. clinical practice. Sleep Med Rev. 2009; 13(4): 265–274. [DOI] [PubMed] [Google Scholar]
- 4. Davidson JR, Aime A, Ivers H, Morin CM. Characteristics of individuals with insomnia who seek treatment in a clinical setting versus those who volunteer for a randomized controlled trial. Behav Sleep Med. 2009; 7(1): 37–52. [DOI] [PubMed] [Google Scholar]
- 5. Stepanski E, Koshorek G, Zorick F, Glinn M, Roehrs T, Roth T. Characteristics of individuals who do or do not seek treatment for chronic insomnia. Psychosomatics. 1989; 30(4): 421–427. [DOI] [PubMed] [Google Scholar]
- 6. Krakow B, Ulibarri VA, Romero EA. Patients with treatment-resistant insomnia taking nightly prescription medications for sleep: a retrospective assessment of diagnostic and treatment variables. Prim Care Companion J Clin Psychiatry. 2010; 12(4): 1–12. doi:10.4088/PCC.09m00873bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Psychiatrc Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5TM (5th Ed.). Arlington, VA: American Psychiatric Publishing, Inc; 2013. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2013-14907-000&site=ehost-live Accessed April 1, 2016. [Google Scholar]
- 8. Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995; 18(7): 581–588. http://www.ncbi.nlm.nih.gov/pubmed/8552929 Accessed October 31, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001; 86(8): 3787–3794. [DOI] [PubMed] [Google Scholar]
- 10. Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep. 2011; 34(12): 1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Randall S, Roehrs TA, Roth T. Efficacy of eight months of nightly zolpidem: a prospective placebo-controlled study. Sleep. 2012; 35(11): 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007; 30(8): 959–968. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1978384&tool=pmcentrez&rendertype=abstract Accessed April 20, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scharf MB, Black J, Hull S, Landin R, Farber R. Long-term nightly treatment with indiplon in adults with primary insomnia: results of a double-blind, placebo-controlled, 3-month study. Sleep. 2007; 30(6): 743–752. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1978349&tool=pmcentrez&rendertype=abstract Accessed April 27, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998; 158(10):1099–1107. http://www.ncbi.nlm.nih.gov/pubmed/9605781 Accessed April 27, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Lichstein K, Taylor DJ, McCrae CS, Ruiter ME. Insomnia: epidemiology and risk factors. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine (Fifth Edition). Philadelphia, PA: W.B. Saunders; 2011: 827–837. doi:http://dx.doi.org/10.1016/B978-1-4160-6645-3.00071-2. [Google Scholar]
- 16. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002; 6(2): 97–111. [DOI] [PubMed] [Google Scholar]
- 17. U.S. Food and Drug Administration. Lunesta (eszopiclone) Tablets: Safety Labeling Changes Approved By FDA Center for Drug Evaluation and Research (CDER) 2008. http://www.fda.gov.proxy.lib.umich.edu/Safety/MedWatch/SafetyInformation/Safety-RelatedDrugLabelingChanges/ucm106688.htm Accessed April 1, 2016.
- 18. U.S. Food and Drug Administration. AMBIEN: Highlights of Prescribing Information 2004. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/019908s035,021774s016lbl.pdf Accessed April 1, 2016.
- 19. Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006; 59(11): 1052–1060. [DOI] [PubMed] [Google Scholar]
- 20. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011; 72(7): 914–928. [DOI] [PubMed] [Google Scholar]
- 21. Asnis GM, Chakraburtty A, DuBoff EA, et al. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry. 1999; 60(10): 668–676. http://www.ncbi.nlm.nih.gov/pubmed/10549683 Accessed April 27, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Pollack M, Kinrys G, Krystal A, et al. Eszopiclone coadministered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Arch Gen Psychiatry. 2008; 65(5): 551–562. [DOI] [PubMed] [Google Scholar]
- 23. Pollack MH, Hoge EA, Worthington JJ, et al. Eszopiclone for the treatment of posttraumatic stress disorder and associated insomnia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011; 72(7): 892–897. [DOI] [PubMed] [Google Scholar]
- 24. Fava M, Asnis GM, Shrivastava R, et al. Zolpidem extended-release improves sleep and next-day symptoms in comorbid insomnia and generalized anxiety disorder. J Clin Psychopharmacol. 2009; 29(3): 222–230. [DOI] [PubMed] [Google Scholar]
- 25. Walsh JK, Muehlbach MJ, Lauter SA, Hilliker NA, Schweitzer PK. Effects of triazolam on sleep, daytime sleepiness, and morning stiffness in patients with rheumatoid arthritis. J Rheumatol. 1996; 23(2): 245–252. http://www.ncbi.nlm.nih.gov/pubmed/8882027 Accessed April 24, 2016. [PubMed] [Google Scholar]
- 26. Roth T, Price JM, Amato DA, Rubens RP, Roach JM, Schnitzer TJ. The effect of eszopiclone in patients with insomnia and coexisting rheumatoid arthritis: a pilot study. Prim Care Companion J Clin Psychiatry. 2009; 11(6): 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soares CN, Joffe H, Rubens R, Caron J, Roth T, Cohen L. Eszopiclone in patients with insomnia during perimenopause and early postmenopause: a randomized controlled trial. Obstet Gynecol. 2006; 108(6): 1402–1410. [DOI] [PubMed] [Google Scholar]
- 28. Joffe H, Petrillo L, Viguera A, et al. Eszopiclone improves insomnia and depressive and anxious symptoms in perimenopausal and postmenopausal women with hot flashes: a randomized, double-blinded, placebo-controlled crossover trial. Am J Obstet Gynecol. 2010; 202(2): 171.e1–171.e11. [DOI] [PubMed] [Google Scholar]
- 29. Goforth HW, Preud’homme XA, Krystal AD. A randomized, double-blind, placebo-controlled trial of eszopiclone for the treatment of insomnia in patients with chronic low back pain. Sleep. 2014; 37(6): 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014; 37(2): 343–349. doi:10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balkrishnan R, Rasu RS, Rajagopalan R. Physician and patient determinants of pharmacologic treatment of sleep difficulties in outpatient settings in the United States. Sleep. 2005; 28(6): 715–719. http://www.ncbi.nlm.nih.gov/pubmed/16477958 Accessed April 28, 2016. [DOI] [PubMed] [Google Scholar]
- 32. Moloney ME, Konrad TR, Zimmer CR. The medicalization of sleeplessness: a public health concern. Am J Public Health. 2011; 101(8): 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walsh JK. Drugs used to treat insomnia in 2002: regulatory-based rather than evidence-based medicine. Sleep. 2004; 27(8): 1441–1442. http://www.ncbi.nlm.nih.gov/pubmed/15683131 Accessed April 28, 2016. [DOI] [PubMed] [Google Scholar]
- 34. Barbone F, McMahon AD, Davey PG, et al. Association of road-traffic accidents with benzodiazepine use. Lancet (London, England). 1998; 352(9137): 1331–1336. http://www.ncbi.nlm.nih.gov/pubmed/9802269 Accessed April 28, 2016. [DOI] [PubMed] [Google Scholar]
- 35. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012; 345: e6231 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3460255&tool = pmcentrez&rendertype = abstract Accessed April 28, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moore N, Pariente A, Bégaud B. Why are benzodiazepines not yet controlled substances? JAMA Psychiatry. 2015; 72(2): 110–111. [DOI] [PubMed] [Google Scholar]
- 37. Ohayon MM, Caulet M, Arbus L, et al. Are prescribed medications effective in the treatment of insomnia complaints? J Psychosom Res. 1999; 47(4): 359–368. http://www.ncbi.nlm.nih.gov/pubmed/10616230 Accessed April 24, 2016. [DOI] [PubMed] [Google Scholar]
- 38. Balter MB, Uhlenhuth EH. The beneficial and adverse effects of hypnotics. J Clin Psychiatry. 1991; 52(Suppl): 16–23. http://www.ncbi.nlm.nih.gov/pubmed/2071567 Accessed April 24, 2016. [PubMed] [Google Scholar]
- 39. Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009; 25(10): 2487–2494. [DOI] [PubMed] [Google Scholar]
- 40. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011; 34(5): 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001; 2(4): 297–307. http://www.ncbi.nlm.nih.gov/pubmed/11438246 Accessed February 24, 2016. [DOI] [PubMed] [Google Scholar]
- 42. IBM SPSS Statistics for Windows, Version 23.0. 2015.
- 43. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989; 129(1): 125–137. [DOI] [PubMed] [Google Scholar]
- 44. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders. Biol Psychiatry. 2011; 69(6): 592–600. doi:10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 45. Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003; 41(4): 427–445. http://www.ncbi.nlm.nih.gov/pubmed/12643966 Accessed October 31, 2015. [DOI] [PubMed] [Google Scholar]
- 46. Moul DE, Hall M, Pilkonis PA, Buysse DJ. Self-report measures of insomnia in adults: rationales, choices, and needs. Sleep Med Rev. 2004; 8(3): 177–198. [DOI] [PubMed] [Google Scholar]
- 47. Drake CL, Vargas I, Roth T, Friedman NP. Quantitative measures of nocturnal insomnia symptoms predict greater deficits across multiple daytime impairment domains. Behav Sleep Med. 2015; 13(1): 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. NIH State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults. J Clin Sleep Med. 2005; 1(4): 412–421. http://www.ncbi.nlm.nih.gov/pubmed/17564412 Accessed October 11, 2015. [PubMed] [Google Scholar]
- 49. Krystal AD, McCall WV, Fava M, et al. Eszopiclone treatment for insomnia: effect size comparisons in patients with primary insomnia and insomnia with medical and psychiatric comorbidity. Prim care companion CNS Disord. 2012; 14(4): 1–31. doi:10.4088/PCC.11m01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014; 13(5): 461–471. [DOI] [PubMed] [Google Scholar]
- 51. Herring WJ, Connor KM, Ivgy-May N, et al. Suvorexant in patients with i: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016; 79(2): 136–148. [DOI] [PubMed] [Google Scholar]
- 52. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011; 34(10): 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008; 4(5): 487–504. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 2576317&tool = pmcentrez&rendertype = abstract Accessed April 12, 2016. [PMC free article] [PubMed] [Google Scholar]
- 54. Krystal AD. Pharmacologic treatment of insomnia: other medications. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine (Sixth Edition). 6th ed. St. Louis, MO: Elsevier; 2016: 842–854. [Google Scholar]
- 55. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015; 163(3): 191–204. [DOI] [PubMed] [Google Scholar]
- 56. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016; 165: 125–133. doi:10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 57. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999; 22(Suppl 2): S347–S353. [PubMed] [Google Scholar]
- 58. O’Keefe DJ. Colloquy: should familywise alpha be adjusted?: against familywise alpha adjustment. Hum Commun Res. 2003; 29(3): 431–447. doi:10.1093/hcr/29.3.431. [Google Scholar]
- 59. Kaufmann CN, Spira AP, Alexander GC, Rutkow L, Mojtabai R. Trends in prescribing of sedative-hypnotic medications in the USA: 1993–2010. Pharmacoepidemiol Drug Saf. 2016; 25: 637–645. doi:10.1002/pds.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011; 31(2): 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011; 135(1–3): 10–19. [DOI] [PubMed] [Google Scholar]
- 62. Li Y, Vgontzas AN, Fernandez-Mendoza J, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension. 2015; 65(3): 644–650. [DOI] [PubMed] [Google Scholar]
- 63. Sivertsen B, Lallukka T, Salo P, et al. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT Study in Norway. J Sleep Res. 2014; 23(2): 124–132. [DOI] [PubMed] [Google Scholar]
- 64. Canivet C, Nilsson PM, Lindeberg SI, Karasek R, Östergren PO. Insomnia increases risk for cardiovascular events in women and in men with low socioeconomic status: a longitudinal, register-based study. J Psychosom Res. 2014; 76(4): 292–299. [DOI] [PubMed] [Google Scholar]
- 65. Freeman D, Sheaves B, Goodwin GM, et al. Effects of cognitive behavioural therapy for insomnia on the mental health of university students: study protocol for a randomized controlled trial. Trials. 2015; 16: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gosling JA, Glozier N, Griffiths K, et al. The GoodNight study–online CBT for insomnia for the indicated prevention of depression: study protocol for a randomised controlled trial. Trials. 2014; 15: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]