Abstract
OA is a multifaceted and heterogeneous syndrome that may be amenable to tailored treatment. There has been an increasing focus within the OA research community on the identification of meaningful OA phenotypes with potential implications for prognosis and treatment. Experimental and clinical data combined with sophisticated statistical approaches have been used to characterize and define phenotypes from the symptomatic and structural perspectives. An improved understanding of the existing phenotypes based on underlying disease mechanisms may shed light on the distinct entities that make up the disease. This narrative review provides an updated summary of the most recent advances in this field as well as limitations from previous approaches that can be addressed in future studies.
Keywords: osteoarthritis, phenotype, treatment, classification
Rheumatology key messages
OA is a complex syndrome rather than a single disease.
OA subgroups can be characterized based on differences in prognosis, therapeutic response or disease mechanisms.
OA phenotyping includes the identification of key phenotypic characteristics, appropriate statistical approaches and extensive validation.
Introduction
OA is characterized by an active and complex process involving inflammatory, mechanical and metabolic factors, which ultimately lead to the structural destruction and failure of the synovial joint [1]. Virtually all joint tissues may be affected in OA, particularly in late stages of the disease, including the hyaline articular cartilage, subchondral bone, synovium and soft-tissue structures, such as ligaments, muscle and menisci [2]. Pain is the overriding symptom in persons with knee OA and a major driver of clinical decision-making, but other issues, such as difficulty in performing activities, joint stiffness and mood and sleep disturbances, are also commonly reported by patients during medical encounters [3].
It has long been noticed that there is great variability in the clinical presentation and long-term disease prognosis across patients with OA [4, 5]. It has been >30 years since the label mixed bag of disorders was used to describe the heterogeneity of OA [4] and, although several advances have occurred since that time, OA heterogeneity still remains a contemporaneous challenge in clinical practice and research [6]. The wide range of risk factors that have been associated with OA, such as older age, obesity, joint injury (including meniscal and ligament tears), biomechanical factors such as joint shape and alignment, hormonal changes, metabolic disturbances and genetic predisposition, indicates that there may be multiple underlying pathways leading to similar outcomes of joint destruction [7–9]. In this context, OA can be seen as a syndrome rather than a single disease [10, 11]. Although a disease is usually defined as an entity associated with a specific cause and specific anatomical or functional abnormalities, a syndrome is characterized by similar signs and symptoms with different causes and manifestations [12]. Nevertheless, a clear definition of the existing OA subtypes has still not emerged, although it is likely to be forthcoming in the near future.
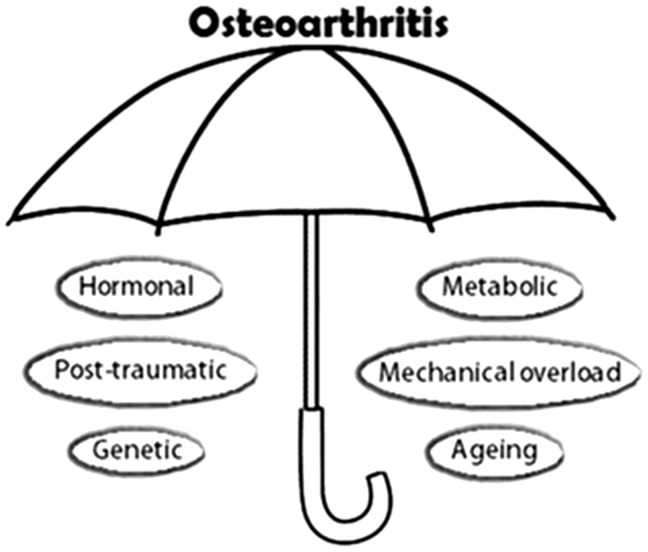
In the past, the heterogeneity of OA was described by a disease classification system into idiopathic and secondary types and according to the joint site [9]. However, as the knowledge of the disease pathogenesis has evolved substantially in the past two decades, specific aetiologies have been identified for most of the formerly called idiopathic (or primary) OA, making this classification problematical and obsolete [13]. There is currently an identifiable predisposing factor for most forms of OA, whether this is oestrogen deficiency [14], ageing [15] or other potential causes mentioned above (Fig. 1). The contemporary OA definition that relies solely on cardinal clinical features and identification of tibiofemoral osteophytes and/or joint space narrowing on the plain radiograph [16] may conceal the range of potential pathways leading to the ultimate syndrome of OA and does not allow for risk stratification and targeted treatment.
Fig. 1.
Distinct OA risk factors and possible mechanistic phenotypes
For many years, patients’ particular characteristics were not taken into consideration in the management of OA. There were few possible options in the therapeutic armamentarium and limited evidence that the therapeutic impact could vary significantly in different types of patients. It was not until more recently that the development of a number of novel potential pharmacological and non-pharmacological treatments with diverse mechanisms of actions has led to the need to define homogeneous groups to tailor the treatment according to specific OA subtypes and achieve better treatment outcomes [17, 18].
Towards precision medicine for OA: how would phenotyping help?
Over the past two decades, a better understanding of the pathobiology of OA as well as mechanisms potentially associated with the perception of pain, including altered pain processing at the joint and brain levels, has emerged [19, 20]. This has provided new targets for potential OA management, in terms of both symptomatic relief and long-term disease modification. However, despite increasing efforts to develop new therapies and expand the therapeutic armamentarium, current interventions for OA are often only marginally effective at decreasing pain, and a significant proportion of patients remain vulnerable to the consequences of the disease, such as progressive pain and loss of functionality [17].
There are many reasons for the slow progress in OA research, including difficulties in detecting disease in pre-symptomatic stages, the slowly progressive nature of OA and insensitive measures of disease activity [21]. However, a major limitation, particularly in clinical trials, is that people with OA resulting from different aetiologies and with differences in the pattern of joint involvement are often lumped together despite the significant heterogeneity across them [6, 10, 21]. Research into the pathogenesis of OA is revealing that each of the common OA risk factors may take a different mechanistic pathway to OA, such that the mediators that promote the development of OA in older adults may be different from those that promote OA after a joint injury in a younger adult or in someone who is obese.
From a symptomatic perspective, there is a need to stratify patients for treatment according to the key factors influencing the perception of pain in each patient, whether this is structural joint pathology, psychosocial issues or sensitization of pain pathways [22, 23]. It has been demonstrated that several articular and periarticular structures are able to generate pain in knee OA patients, such as synovitis, subchondral bone marrow lesions, periarticular muscle dysfunction and bursitis [23, 24]. In contrast, higher levels of anxiety and depression have been associated with increased OA pain [25]. However, the presence and severity of these features vary considerably across the whole knee OA population [26, 27], and their contribution to symptoms is likely to differ among individuals or subgroups of individuals with OA.
It is widely recognized that there is a great dissociation between the intensity of symptoms and the severity of the structural damage on imaging. Most of the studies to date have investigated pain phenotypes and phenotypes of structural damage as discrete entities [28]. It is likely that there are different key features associated with phenotypes from each perspective (symptoms vs structure) and that clinically relevant phenotypes of OA pain may not be as relevant for structural outcomes or trials investigating disease-modifying OA drugs and vice versa.
A number of previous reviews have discussed the feasibility and advantages of drug and non-drug therapies for OA targeted to particular patient subgroups or phenotypes and are highly recommended reading complementary to this narrative review [6, 10, 17, 18, 21, 22, 29].
Evidence supporting the existence of phenotypes
A phenotype refers to a composite of observable characteristics or traits of an individual that results from genetic and environmental factors, whereas an endotype refers to a subtype of disease defined functionally and pathologically by a molecular mechanism or by a treatment response [30]. McInnes et al. [31] have recently proposed the concept of using endotypes to select patients with RA who are most likely to benefit from a specific anti-cytokine therapy. Likewise, identification of OA endotypes will be useful in advancing therapies targeted to specific mechanisms underlying the pathogenesis of OA.
Three main possible approaches have been described for stratifying patients into relevant subgroups in the field of back pain [32], which are also applicable in the context of OA: phenotypes based on underlying disease mechanisms (mechanistic phenotypes or endotypes); based on disease prognosis; and based on response to therapies (Table 1). Although it is possible that distinct subgroups will exist depending on which approach is pursued, it is likely that there is significant overlap between them. It is intuitive that distinct phenotypes based on similar pathogenic processes will also respond in a similar manner to therapies and experience similar patterns of structural damage progression. However, there is a lack of robust data in the literature so far to support this assumption. For the purpose of this review, we give examples of studies that provided evidence of the existence of discrete subgroups using each one of the approaches outlined above and describe particular aspects of each approach.
Table 1.
Phenotype categorization in OA and examples in each phenotype category
| Mechanistic subgroups | Prognosis | Response to therapy |
|---|---|---|
| Inflammatory OA | Disease stage | Disease stage |
| Cell senescence | Pain intensity | Type of pain (e.g. neuropathic vs non-neuropathic) |
| Mechanical overload | Mechanical factors (obesity, malalignment) | Synovitis/effusion |
| Metabolic | Contra-lateral knee OA | Subchondral bone lesions |
| Genetic | Family history | Gender |
| Oestrogen deficiency | Knee injury | Presence of co-morbid conditions |
| Single vs multi-joint OA | Single vs multi-joint OA |
Mechanistic subgroups
Several studies have stratified OA patients based on specific pathological processes from different perspectives and using different approaches [28, 33]; however, the relevance of these specific subgroups in the context of randomized clinical trials (RCTs) is still not well established in the OA literature. Moreover, most of these stratifications examined a single feature involved in the OA pathogenesis (e.g. phenotyping patients by subchondral bone characteristics [34]), and very few integrated multiple dimensions to develop, and subsequently test, a comprehensive classification system. Therefore, results from current studies investigating phenotypes should be interpreted as exploratory, as no OA phenotype at present has been properly validated across populations and against important outcomes.
The existence of a phenotype with an increased inflammatory component has been investigated in several studies. Attur et al. [35] identified two distinct subgroups of symptomatic knee OA patients with different profiles of inflammatory gene expression in peripheral blood leucocytes using cluster analysis (see section below on Methodological aspects of previous approaches: strengths and limitations). Pro-inflammatory cytokines, such as IL-1β, were significantly elevated in the inflammatory phenotype. Individuals in this group had higher pain levels, decreased physical function and greater rates of joint space narrowing on radiographs over 2 years compared with the non-inflammatory phenotype and controls. In another recent study, a subgroup with greater knee synovitis severity was identified by histopathological analysis including individuals at post-mortem and patients undergoing knee arthroplasty [36]. The synovitis severity score was the main feature that distinguished the two subgroups of patients with established OA who had similar clinical and demographic characteristics.
Several other potential mechanistic phenotypes have been proposed, such as a metabolic phenotype, previously investigated using both clinical [37, 38] and metabolic marker data [39], and a phenotype related to cell senescence. Senescent cells have been shown to produce high levels of pro-inflammatory cytokines and matrix-degrading enzymes that promote tissue destruction. Interventions that specifically target and destroy senescent cells (senolytics) are being developed, and identification of a senescent endotype would greatly enhance the chance of success for a senolytic in a clinical trial. As proof of concept, in a recent preclinical study, mice with post-traumatic OA and age-related OA were found to develop less severe disease when senescent cells were deleted [40], suggesting that cell senescence contributes to more than one OA phenotype. Although there seems to be sufficient information suggesting the existence of mechanistic phenotypes, their relevance for important OA outcomes, such as prognosis and response to therapies, is yet to be clarified.
Pain subgroups
In addition to mechanistic phenotypes of structural damage, multiple studies have investigated pain phenotypes in knee OA [41–47]. Kittelson et al. [42] integrated a broad range of clinical characteristics related to the knee pain experience and identified four pain phenotypes with possibly different mechanisms contributing to pain. They were characterized by: higher number of co-morbidities (a phenotype of older adults); greater knee pain sensitivity; higher psychological distress; and a phenotype including 62% of the population, characterized by less severe radiographic OA and lower involvement of all other pain features. Radiography was the only parameter included representing structural joint pathology, and it is unknown whether there were further differences in other pain-relevant structural features not revealed by radiography (e.g. bone marrow lesions, synovitis, denuded bone areas and soft tissue involvement), particularly in this last, less characterized phenotype. This is relevant in terms of personalized symptomatic treatment, as individuals with mild radiographic OA might possibly benefit from symptomatic treatments targeting the specific knee structural pathology, whereas individuals with more severe joint OA might also need interventions addressing chronic pain sensitization and psychological distress.
Prognostic subgroups
A great proportion of knee OA patients do not experience significant progression of joint space narrowing over several years [48, 49], which is the current gold-standard method for assessing clinical efficacy in OA trials investigating disease-modifying OA drugs. Therefore, a great interest exists in identifying biomarkers of disease progression or phenotypes of patients who are more likely to experience a progressive course in order to optimize the inclusion of patients in trials. However, to date no single marker has been found to be sufficient for diagnosis or prognosis in OA, underscoring the view that phenotypes might be more related to prognostic outcomes than single biomarkers.
Phenotypes associated with structural progression were investigated using combined individual patient data from two RCTs investigating the effect of calcitonin in symptomatic knee OA patients [48]. The combination of Kellgren–Lawrence grade (KLG) with pain severity had a stronger association with progression compared with KLG alone, suggesting that structural progression is mainly driven by patient-specific phenotype rather than disease stage. In another recent study [50], a prognostic prediction rule for rapidly progressive radiographic tibiofemoral OA in individuals with KLG 0 or 1 was developed using radiographs and clinical variables. The study used data from the Multicentre Osteoarthritis Study and Osteoarthritis Initiative (OAI) datasets, two large community-based studies. The best set of predictors for developing KLG 3 or 4 OA within 5 years included contralateral knee OA, a baseline index knee OA grade of 1, higher BMI and higher baseline WOMAC total scores. It is important to note that patients sharing similar rates of progression do not necessarily share other characteristics that would group them within the same phenotype from a mechanistic or a therapeutic perspective [51].
Subgroups based on response to therapy
Results from RCTs generally reflect the average treatment effect in the study population as a whole, which does not take into account heterogeneous responses to treatment across the patients. If a particular subgroup of patients experience benefits from a given treatment but another subgroup do not, the result of the trial may be largely negative if the subgroup with positive response is not identified properly. In this context, RCTs are necessary for the assessment of treatment efficacy in specific subgroups of patients. Other study designs, such as cohorts, may identify predictors of outcome in general (regardless of the treatment received), instead of truly identifying treatment effect modifiers or predictors of treatment response [32]. Nevertheless, shortcomings exist concerning subgroup analyses, such as the possibility of false positives when multiple comparisons are tested without a prespecified hypothesis and inadequate power to detect an effect in a particular subgroup [52, 53]. This was illustrated in a landmark trial in cardiology investigating the effect of aspirin in acute myocardial infarction by patients’ astrological birth sign [54]. Although aspirin had a highly significant benefit over placebo on vascular mortality, Gemini and Libra patients did not experience the same reduction in mortality as the other zodiac sign groups [55]. The subgroup effect, although statistically significant, was not endorsed by the authors, but the results were published to warn about the risk of misleading results from subgroup analysis and need for careful interpretation of subgroup claims in clinical trials. More recently, easy-to-apply criteria have been proposed to aid in the assessment of credibility of subgroup effects [53, 56].
Efforts are underway in the OA field to perform methodologically sound subgroup analyses in order to assess the efficacy of a number of interventions in specific and pre-determined subgroups of patients who might have better response to a given treatment. In 2010, the OA Trial Bank was initiated to accomplish this purpose by gathering individual patient data from existing RCTs in OA. This initiative provides a unique opportunity to combine data from a large number of patients recruited from multiple centres in various countries, which is more likely to be representative of the broader OA population than single-centre studies.
A recent meta-analysis embedded in the OA Trial Bank investigated the effects of IA glucocorticoid injection in patients with knee or hip OA. The meta-analysis included seven trials (a total of 620 patients) and revealed that patients with severe pain (⩾70 on 0–100 scale) had significantly greater improvement in pain in the short term (up to 4 weeks) compared with patients with less pain, although no difference was found on mid- and long-term follow-ups. The study also showed that there was no influence of inflammatory signs, detected either by clinical examination or by ultrasound, in the magnitude of response [57]. Other therapies are being investigated using the same methodology, including oral glucosamine, exercise and topical therapies (NSAID and capsaicin) [58].
Methodological aspects of previous approaches: strengths and limitations
There is a rapidly growing number of studies aimed to identify and characterize distinct subgroups of knee OA [33]. In this section, we highlight some of the methodological differences as well as important limitations in these studies and provide an abridged framework for future studies aimed to identify a comprehensive classification of phenotypes.
Most studies to date have focused on particular disease attributes separately (e.g. causal factors, imaging findings, sensitivity to pain) and have found subgroups according to these different predefined perspectives. In addition, whereas some studies hypothesized subgroups based on the observation of a restricted number of variables (e.g. atrophic vs hypertrophic OA on imaging [59]), others used specific data-driven methods (e.g. cluster analysis) to identify subgroups. Data-driven approaches are considered a more reliable and appropriate method of deriving subgroups, particularly when complex subgroups may be present [51], as they do not require an a priori hypothesis of what the subgroups are. However, they are highly dependent on the choice of variables included in the analysis and require extensive validation.
The key steps involved in the study of phenotypes are outlined in Fig. 2. These involve the identification of the most promising phenotypic characteristics, selection of an appropriate statistical approach and extensive validation of the findings.
Fig. 2.
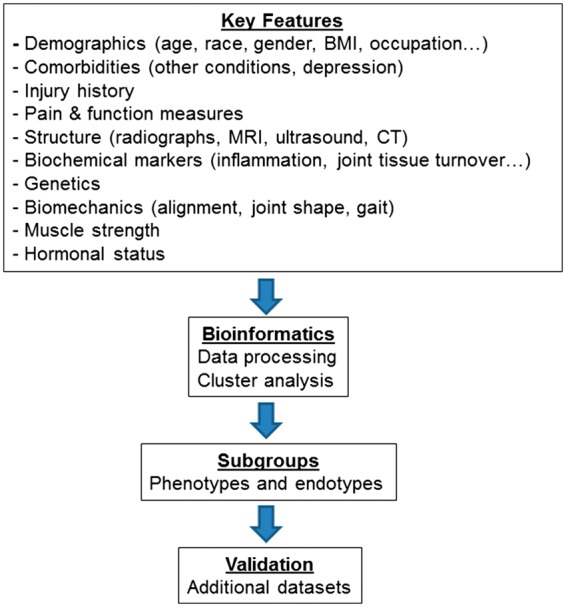
Workflow for OA phenotyping
Key OA features potentially associated with phenotypes
Identifying the key features that should be considered in defining OA phenotypes is a paramount step to define an accurate classification of phenotypes, link phenotypes to underlying mechanisms (i.e. define OA endotypes) and use this information to inform clinical studies. In this context, several factors may be common to more than one phenotype, whereas others may not be important enough to distinguish patients into subgroups that have implications for outcomes. Therefore, there is a crucial need to identify the OA characteristics that are more associated with the interpatient variability in clinical presentation and important outcomes, such as treatment response and prognosis.
The Initiative on Methods, Measurement and Pain Assessment in Clinical Trials group recently published evidence-based recommendations on the core phenotypic domains as well as characteristics in each domain for use in future studies aimed to identify patient subgroups with different response to analgesic treatments [60]. As yet, there is a lack of such recommendations in the OA field. The specific roles of key OA domains, including clinical, biomechanical, genetic, imaging and laboratory, in the delineation of phenotypes from the symptomatic and structural perspectives are yet to be determined, as well as the most promising characteristics within each domain. More solid evidence-based recommendations to elucidate this issue would greatly benefit this field of research and provide guidance for future studies. In 2011, the Osteoarthritis Research Society International highlighted the need to improve the understanding of the subgroups of OA and that a comprehensive definition was needed [61].
To shed light on this question, we recently conducted a systematic review focused on knee OA subgroup/phenotype studies assessing the association of the subgroups with clinical and/or structural outcomes [28]. The review included 34 studies and indicated that clinical phenotypes were more often investigated, whereas there were fewer studies investigating structural phenotypes, especially using imaging data. Based on the included studies, it was found in more than one study that phenotypes based on differences in sensory and psychological profile, presence of co-morbid symptoms such as fatigue and pain in other body sites, muscle strength and radiographic severity were associated with distinct pain and/or function outcomes. Stratification of patients based on the biochemical biomarker profile (of inflammation, bone and cartilage metabolism) was predominantly associated with different structural outcomes, such as radiographic severity (KLG) and rate of cartilage volume loss, except for a subgroup with a higher inflammatory component, which also presented poorer clinical outcomes [35, 49, 62]. BMI, metabolic profile and inflammation were, in general, associated with both clinical and structural outcomes.
Furthermore, additional baseline factors were found to be associated with subgroups defined based on different trajectories of progression. Demographic characteristics, such as age, gender, race, BMI, education and social class, were related to unfavourable trajectories of clinical progression, whereas alcohol use, smoking status and presence of hand OA had no association with clinical trajectories. Age, BMI and gender were also linked to distinct trajectories of radiographic progression, with male patients significantly outnumbering females in the small subgroup comprising 2% of the population who experienced the greatest decline in joint space width over 2 years [49].
Analytical approaches for subgrouping
Cluster analysis is one of the most commonly used subgrouping analytical approaches in the OA field [28]. Individuals are grouped according to similarities in the variables included in the model in a way that individuals within a subgroup share more similarities with each other than with individuals in another subgroup [63]. There are different clustering algorithms within the broader concept of cluster analysis, such as k-means and hierarchical methods, each using particular methods to determine the optimal number of clusters and to handle the variables in order to define the clusters [64]. In contrast, in latent class analysis, another subgrouping approach increasingly used in OA research [36, 42, 65, 66], the distribution of the variables is first described and subsequently used to assess the probability of being unique to each latent class. There are a variety of other possible data-driven approaches for the identification of subgroups, such as factor analysis and self-organizing maps. These are called unsupervised techniques, where subgroups are derived based on the relationships between variables using cross-sectional data. A crucial step in these analyses is to test whether the subgroups are associated with clinically meaningful outcomes in order to determine their clinical relevance. A second methodological approach is the supervised techniques, where subgroups are modelled by aligning with a prespecified outcome using longitudinal data. The advantage of the supervised techniques is that the subgroups have instant face validity as they are defined based on their differences in a given outcome. However, a drawback of this approach is that the subgroups identified are typically dependent on a single outcome, and several distinct classifications of subgroups may exist depending on the outcome of interest. A more detailed discussion on the differences between these approaches can be found elsewhere [67].
In our view, a sensible approach would be to integrate the key OA factors involved in the OA heterogeneity (possibly including risk factors, clinical features, imaging and biomarkers), using an appropriate statistical model, in order to identify homogeneous subgroups which should then be tested against clinically important outcomes and validated in external cohorts and clinical trials (see next subsection; Fig. 2). It is likely that several factors driving the structural and symptomatic disease will be common to more than one phenotype, whereas others may be more prominent in specific phenotypes. In our limited experience, latent class analysis seems to have several advantages over cluster analysis, because it uses probabilities to identify the most similar patterns within the dataset and is able to handle missing data and to accommodate different types of variables (e.g. categorical, continuous) [68]. However, more evidence-based guidance on the optimal methods as well as the underlying assumptions for the most common research questions in the context of OA would be helpful. A similar research methods framework has been published for subgrouping of low back pain [67].
Testing the stability and validity of the phenotypes
Testing the robustness of the findings and validity of the phenotypes (both internally and externally) are other important steps in defining a reliable phenotypic classification. Firstly, there are a number of indices to assess model fit and aid in the decision of the optimal number of subgroups. These should be reported by the studies using data-driven techniques, along with measures that assess the certainty of a patient’s subgroup membership, such as entropy and posterior probabilities [68].
Secondly, evaluating the stability of the phenotypes is an important step in order to test the robustness of the findings when changes are made to the dataset [69]. This can be tested easily; for example, by dividing the dataset into halves and repeating the same analysis in each new dataset in order to confirm whether equivalent phenotypes will be identified. Moreover, it is recommended that similar phenotypes should be present when distinct time points are used, which would support that the phenotype's classification is also stable over time.
Moreover, phenotypes will be important for use in clinical research and practice only if they are clearly relevant for important OA outcomes, such as prognosis and response to treatment. Thus, mechanistic phenotypes should be tested for their association with clinical and/or structural outcomes, ideally using longitudinal outcomes and, ultimately, the investigation of whether treatment response differs in the proposed phenotypes. An important finding from our recent systematic review on studies investigating knee OA phenotypes was that most of the studies had a cross-sectional design, and very few used longitudinal outcomes to determine the clinical relevance of the phenotypes [28].
Finally, as noted above, it is recommended that studies investigating phenotypes should include individuals representative of the entire knee OA population, particularly involving patients recruited from multiple centres and locations [51]. In OA, this could possibly be accomplished by merging data from large community-based cohorts, such as the OAI and the Multicentre Osteoarthritis Study. Several studies to date have included specific populations, such as patients scheduled for knee arthroplasty, which limits the generalizability of the findings [28]. In addition, it is crucial to assess whether the phenotypes are also valid in different populations (external validity). As an example of this, Knoop et al. [41] identified five phenotypes using limited clinical data from the OAI, which were minimal joint disease, strong muscle, non-obese and weak muscle, obese and weak muscle, and depressive phenotype. These phenotypes were further investigated in a subsequent study using data from an Amsterdam OA cohort [70], which found very similar phenotypes using the same clinical data and clustering approach.
Challenges and opportunities for future research
Several challenges exist in defining phenotypes for use in clinical practice and research. OA is a highly heterogeneous condition, and the understanding of which features are common to multiple phenotypes and the ones that distinguish patients into relevant subgroups is still a great research challenge. Moreover, the utility of subgroups identified using methods that are costly or not readily available in clinical practice, such as MRI or quantitative sensory testing (used to assess pain sensitization), is questionable. The best surrogates or alternative methods to characterize these subgroups will need to be determined. For example, there has been much interest in defining an inflammatory phenotype in OA studies. Physical examination findings of joint inflammation are not sufficiently sensitive in OA [71]. Imaging (ultrasound and MRI) has been used to detect synovitis as a measure of inflammation and blood concentrations of high-sensitivity CRP and IL-6 and novel indicators, including soluble macrophage markers (CD14 and CD163 in the SF and CD163 in serum), have also been investigated as systemic markers of inflammation [72]. In addition, to be accurate and representative of the heterogeneity present in OA, phenotypes should be simple enough to be useful for widespread use in clinical research and practice.
Few studies in the literature have attempted to define a multidimensional classification of phenotypes, and there is a lack of studies testing the prospective validity of the phenotypes using longitudinal outcomes. In addition, there are surprisingly few studies investigating structural or clinical phenotypes using MRI features, such as bone marrow lesions, meniscal damage and synovitis, in symptomatic knee OA patients. In this context, defining a comprehensive classification and testing its external validity is limited by the availability of variables of interest in the same dataset. For example, coping strategies and pain catastrophizing have been shown to be important factors in the perception of pain [25] and characterization of pain phenotypes [42, 44]. However, these features are not widely available in large community-based studies. The same holds true for potentially useful biochemical markers, such as IL-6, IL-1β and high-sensitivity CRP.
Despite these limitations, efforts are underway to improve the characterization of OA phenotypes using a wide array of factors from multiple domains associated with OA, while making sure that the phenotypes are stable and robust, have statistically and clinically significance, and are also valid across populations. The framework provided in this review may be overly simplistic and does not cover in detail all steps involved in the process of characterizing meaningful OA phenotypes. However, it might serve as a starting point for future undertakings aimed to provide solid evidence-based recommendations for future research in OA phenotyping.
Funding: Supported by the National Institute of Musculoskeletal Arthritis, and Skin Diseases grant P60 AR064166 (R.F.L.).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Liu-Bryan R. Inflammation and intracellular metabolism: new targets in OA. Osteoarthritis Cartilage 2015;23:1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loeser RF, Goldring SR, Scanzello CR, Goldring MB.. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennell KL, Hunter DJ, Hinman RS.. Management of osteoarthritis of the knee. BMJ 2012;345:e4934. [DOI] [PubMed] [Google Scholar]
- 4. Bjelle A. On the heterogeneity of osteoarthritis. Clin Rheumatol 1983;2:111–3. [DOI] [PubMed] [Google Scholar]
- 5. Felson DT. The course of osteoarthritis and factors that affect it. Rheum Dis Clin North Am 1993;19:607–15. [PubMed] [Google Scholar]
- 6. Bierma-Zeinstra SM, Verhagen AP.. Osteoarthritis subpopulations and implications for clinical trial design. Arthritis Res Ther 2011;13:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blagojevic M, Jinks C, Jeffery A, Jordan KP.. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2010;18:24–33. [DOI] [PubMed] [Google Scholar]
- 8. Johnson VL, Hunter DJ.. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 2014;28:5–15. [DOI] [PubMed] [Google Scholar]
- 9. Louati K, Vidal C, Berenbaum F, Sellam J.. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 2015;1:e000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruyère O, Cooper C, Arden N. et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging 2015;32:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Altman R, Asch E, Bloch D. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 12. Pearce JM. Disease, diagnosis or syndrome? Pract Neurol 2011;11:91–7. [DOI] [PubMed] [Google Scholar]
- 13. Felson DT. Identifying different osteoarthritis phenotypes through epidemiology. Osteoarthritis Cartilage 2010;18:601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roman-Blas JA, Castañeda S, Largo R, Herrero-Beaumont G.. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther 2009;11:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loeser RF, Collins JA, Diekman BO.. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kellgren JH, Lawrence JS.. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karsdal MA, Michaelis M, Ladel C. et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage 2016;24:2013–21. [DOI] [PubMed] [Google Scholar]
- 18. Tonge DP, Pearson MJ, Jones SW.. The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthritis Cartilage 2014;22:609–21. [DOI] [PubMed] [Google Scholar]
- 19. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther 2009;11:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karsdal MA, Christiansen C, Ladel C. et al. Osteoarthritis – a case for personalized health care? Osteoarthritis Cartilage 2014;22:7–16. [DOI] [PubMed] [Google Scholar]
- 22. Kittelson AJ, George SZ, Maluf KS, Stevens-Lapsley JE.. Future directions in painful knee osteoarthritis: harnessing complexity in a heterogeneous population. Phys Ther 2014;94:422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunter DJ, Guermazi A, Roemer F, Zhang Y, Neogi T.. Structural correlates of pain in joints with osteoarthritis. Osteoarthritis Cartilage 2013;21:1170–8. [DOI] [PubMed] [Google Scholar]
- 24. Hill CL, Gale DR, Chaisson CE. et al. Periarticular lesions detected on magnetic resonance imaging: prevalence in knees with and without symptoms. Arthritis Rheum 2003;48:2836–44. [DOI] [PubMed] [Google Scholar]
- 25. Somers TJ, Keefe FJ, Godiwala N, Hoyler GH.. Psychosocial factors and the pain experience of osteoarthritis patients: new findings and new directions. Curr Opin Rheumatol 2009;21:501–6. [DOI] [PubMed] [Google Scholar]
- 26. Hill CL, Gale DG, Chaisson CE. et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol 2001;28:1330–7. [PubMed] [Google Scholar]
- 27. Felson DT, Chaisson CE, Hill CL. et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 2001;134:541–9. [DOI] [PubMed] [Google Scholar]
- 28. Deveza LA, Melo L, Yamato TP. et al. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage 2017;25:1926–41. [DOI] [PubMed] [Google Scholar]
- 29. Mobasheri A. The future of osteoarthritis therapeutics: targeted pharmacological therapy. Curr Rheumatol Rep 2013;15:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008;372:1107–19. [DOI] [PubMed] [Google Scholar]
- 31. McInnes IB, Buckley CD, Isaacs JD.. Cytokines in rheumatoid arthritis – shaping the immunological landscape. Nat Rev Rheumatol 2016;12:63–8. [DOI] [PubMed] [Google Scholar]
- 32. Foster NE, Hill JC, O’Sullivan P, Hancock M.. Stratified models of care. Best Pract Res Clin Rheumatol 2013;27:649–61. [DOI] [PubMed] [Google Scholar]
- 33. Dell’Isola A, Allan R, Smith SL, Marreiros SSP, Steultjens M.. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskeletal Disord 2016;17:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steinbeck MJ, Eisenhauer PT, Maltenfort MG, Parvizi J, Freeman TA.. Identifying patient-specific pathology in osteoarthritis development based on MicroCT analysis of subchondral trabecular bone. J Arthroplasty 2016;31:269–77. [DOI] [PubMed] [Google Scholar]
- 35. Attur M, Belitskaya-Lévy I, Oh C. et al. Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum 2011;63:1908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wyatt LA, Moreton BJ, Mapp PI. et al. Histopathological subgroups in knee osteoarthritis. Osteoarthritis Cartilage 2017;25:14–22. [DOI] [PubMed] [Google Scholar]
- 37. Lee S, Kim TN, Kim SH. et al. Obesity, metabolic abnormality, and knee osteoarthritis: a cross-sectional study in Korean women. Mod Rheumatol 2015;25:292–7. [DOI] [PubMed] [Google Scholar]
- 38. Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R. et al. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum 2009;61:1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang W, Likhodii S, Zhang Y. et al. Classification of osteoarthritis phenotypes by metabolomics analysis. BMJ Open 2014;4:e006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeon OH, Kim C, Laberge RM. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 2017;23:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knoop J, van der Leeden M, Thorstensson CA. et al. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res 2011;63:1535–42. [DOI] [PubMed] [Google Scholar]
- 42. Kittelson AJ, Stevens-Lapsley JE, Schmiege SJ.. Determination of pain phenotypes in knee osteoarthritis: a latent class analysis using data from the osteoarthritis initiative. Arthritis Care Res 2016;68:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cardoso JS, Riley JL 3rd, Glover T. et al. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain 2016;157:2104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Egsgaard LL, Eskehave TN, Bay-Jensen AC, Hoeck HC, Arendt-Nielsen L.. Identifying specific profiles in patients with different degrees of painful knee osteoarthritis based on serological biochemical and mechanistic pain biomarkers: a diagnostic approach based on cluster analysis. Pain 2015;156:96–107. [DOI] [PubMed] [Google Scholar]
- 45. Frey-Law LA, Bohr NL, Sluka KA. et al. Pain sensitivity profiles in patients with advanced knee osteoarthritis. Pain 2016;157:1988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cruz-Almeida Y, King CD, Goodin BR. et al. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res 2013;65:1786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Osgood E, Trudeau JJ, Eaton TA. et al. Development of a bedside pain assessment kit for the classification of patients with osteoarthritis. Rheumatol Int 2015;35:1005–13. [DOI] [PubMed] [Google Scholar]
- 48. Karsdal MA, Bihlet A, Byrjalsen I. et al. OA phenotypes, rather than disease stage, drive structural progression – identification of structural progressors from 2 phase III randomized clinical studies with symptomatic knee OA. Osteoarthritis Cartilage 2015;23:550–8. [DOI] [PubMed] [Google Scholar]
- 49. Bartlett SJ, Ling SM, Mayo NE, Scott SC, Bingham CO 3rd. Identifying common trajectories of joint space narrowing over two years in knee osteoarthritis. Arthritis Care Res 2011;63:1722–8. [DOI] [PubMed] [Google Scholar]
- 50. Riddle DL, Stratford PW, Perera RA.. The incident tibiofemoral osteoarthritis with rapid progression phenotype: development and validation of a prognostic prediction rule. Osteoarthritis Cartilage 2016;24:2100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burgel PR, Paillasseur JL, Roche N.. Identification of clinical phenotypes using cluster analyses in COPD patients with multiple comorbidities. Biomed Res Int 2014;2014:420134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saragiotto BT, Maher CG, Moseley AM. et al. A systematic review reveals that the credibility of subgroup claims in low back pain trials was low. J Clin Epidemiol 2016;79:3–9. [DOI] [PubMed] [Google Scholar]
- 53. Sun X, Briel M, Busse JW. et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ 2012;344:e1553. [DOI] [PubMed] [Google Scholar]
- 54. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17, 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988;2:349–60. [PubMed] [Google Scholar]
- 55. Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G.. How to use a subgroup analysis: users’ guide to the medical literature. JAMA 2014;311:405–11. [DOI] [PubMed] [Google Scholar]
- 56. Burke JF, Sussman JB, Kent DM, Hayward RA.. Three simple rules to ensure reasonably credible subgroup analyses. BMJ 2015;351:h5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Middelkoop M, Arden NK, Atchia I. et al. The OA Trial Bank: meta-analysis of individual patient data from knee and hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra-articular glucocorticoids. Osteoarthritis Cartilage 2016;24:1143–52. [DOI] [PubMed] [Google Scholar]
- 58. The OA Trial Bank. Individual patient data meta-analysis in osteoarthritis research. http://www.oatrialbank.com/projects. (13 April 2017, date last accessed).
- 59. Roemer FW, Guermazi A, Niu J. et al. Prevalence of magnetic resonance imaging-defined atrophic and hypertrophic phenotypes of knee osteoarthritis in a population-based cohort. Arthritis Rheum 2012;64:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Edwards RR, Dworkin RH, Turk DC. et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 2016;157:1851–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lane NE, Brandt K, Hawker G. et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 2011;19:478–82. [DOI] [PubMed] [Google Scholar]
- 62. Meulenbelt I, Kloppenburg M, Kroon HM. et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage 2007;15:379–85. [DOI] [PubMed] [Google Scholar]
- 63. Kaufman L, Rousseeuw PJ.. Finding groups in data: an introduction to cluster analysis. Wiley; 2009. [Google Scholar]
- 64. Mooi E, Sarstedt M.. A concise guide to market research. Berlin, Germany: Springer, 2011. [Google Scholar]
- 65. Niu J, Felson DT, Neogi T. et al. Patterns of coexisting lesions detected on magnetic resonance imaging and relationship to incident knee osteoarthritis: The multicenter osteoarthritis study. Arthritis Rheumatol 2015;67:3158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waarsing JH, Bierma-Zeinstra SM, Weinans H.. Distinct subtypes of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology 2015;54:1650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kent P, Keating JL, Leboeuf-Yde C.. Research methods for subgrouping low back pain. BMC Med Res Methodol 2010;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kongsted A, Nielsen AM.. Latent class analysis in health research. J Physiother 2017;63:55–8. [DOI] [PubMed] [Google Scholar]
- 69. Vogt W, Nagel D.. Cluster analysis in diagnosis. Clin Chem 1992;38:182–98. [PubMed] [Google Scholar]
- 70. van der Esch M, Knoop J, van der Leeden M. et al. Clinical phenotypes in patients with knee osteoarthritis: a study in the Amsterdam osteoarthritis cohort. Osteoarthritis Cartilage 2015;23:544–9. [DOI] [PubMed] [Google Scholar]
- 71. Maricar N, Callaghan MJ, Parkes MJ, Felson DT, O’Neill TW.. Clinical assessment of effusion in knee osteoarthritis—a systematic review. Semin Arthritis Rheum 2016;45:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Daghestani HN, Pieper CF, Kraus VB.. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol 2015;67:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]