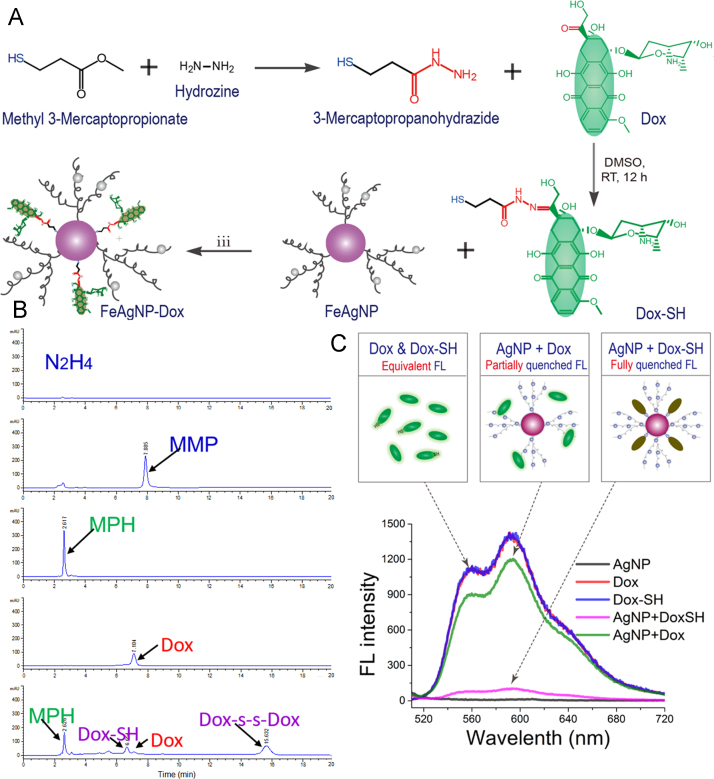
Figure 4.
(A) Schematic illustrations of preparing Dox–SH and Dox-loaded FeAgNPs; (B) HPLC spectrums of the reactants and products. From top to bottom are hydrazine, methyl 3-mercaptopropionate (MMP), and synthesized mercaptopropanohydrazide (MPH, intermediate product) from reaction step (i). Dox, doxorubicin; Dox–SH, final product. (C) Fluorescent spectra of Dox, Dox–SH, and as-prepared FeAgNP-Dox dissolved in ddH2O (λEX = 495 nm, λEM, 510–720 nm, PMT voltage at 400, and split width = 5 nm).