Figure 4. Comparative view of the locations of activating mutations in ER and in AR relative to the position of the ligand, and the relationship of AR mutations to specific AR antagonists.
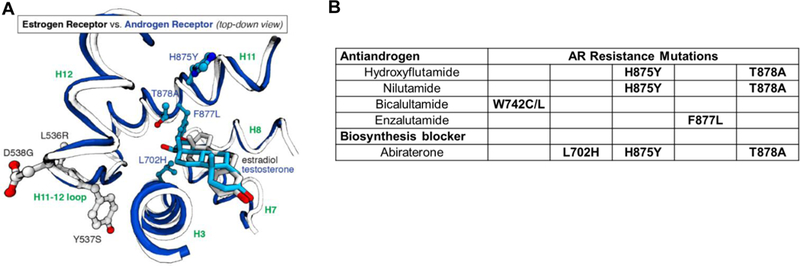
A. A structural overlay of a portion of the LBDs of ER and estradiol (in gray) and testosterone (in blue). The sites of mutation in AR (702, 875, 877, and 878, blue residues) are within the ligand-binding pocket, close to the ligand, whereas the mutation sites in ER (536, 537, and 538, standard atom colored residues) are outside of the pocket, far from ligand contact. (Two rotating 3D movies are available: Supplemental Movie S2 shows that the resistance mutations in the AR LBD are within the LBP. Supplemental Movie S3 shows an overlap comparison of the two LBDs, contrasting the locations of the mutations relative to the ligand.) B. Specific AR mutations are associated with antiandrogens with different structures.64–66 (Because hydroxyflutamide and nilutamide have similar structures, they have similar sites of mutation.) While nominally a blocker of androgen biosynthesis from adrenal precursors, Abiraterone, and particularly its oxidized metabolite D4A, are also direct AR antagonist ligands.118,119 Abiraterone therapy also elevates levels of progestational ligands and suppresses corticosteroid production, necessitating corticosteroid supplementation. These three mutations reduce AR binding specificity and are activated by progestins and corticosteroids.120
