Abstract
Objective:
To describe the perceptions of caregivers of children with medical complexity (CMC) about their decision to pursue tracheostomy for their children, in particular the satisfaction with their decision.
Study design:
In this qualitative study conducted in western North Carolina between 2013–2014, we interviewed 56 caregivers of 41 CMC who had received tracheostomies in the past five years. Three of the CMC were deceased at the time of the interview; 8 were decannulated. In-depth interviews (35 English, 6 Spanish) were conducted, audio-recorded and transcribed verbatim. We used ATLAS.ti software to manage data, and identified themes related to caregiver perceptions about tracheostomy decision.
Results:
We found that caregivers often chose tracheostomy because extending the lives of their children and being able to care for them at home were importantCaregivers reported the many benefits of tracheostomy including improvement in respiratory symptoms, physical and developmental health, quality of life, and means to provide medical care quickly when needed. There were negative effects of tracheostomy such as mucous plugs, excessive secretions, accidental decannulation necessitating emergency tracheostomy tube change, and the increased infection risk. Providing medical care for CMC with tracheostomy at home was difficult, but improved over time. Caregivers were generally satisfied with their decision to pursue tracheostomy for their CMC.
Conclusions:
Decisional satisfaction with tracheostomy for CMC is high. In counseling caregivers about tracheostomy, clinicians should present both the benefits and risks. Future studies should quantify the outcomes described in this study.
Keywords: pediatrics, life-sustaining treatments, decision-making, outcomes
Children with medical complexity (CMC) are a group of children with heterogeneous medical conditions.1 Whereas these conditions once inevitably led to death in the near term, CMC are living longer.2,3 Medical care for CMC often includes life-sustaining treatments (LST), such as tracheostomy, gastrostomy, and chronic mechanical ventilation, with long-term implications for the child’s quality of life, caregiver burden, and caregiver’s quality of life.1,4 Although there has been a steady increase in the number of children receiving tracheostomy in the U.S.,5 there is very limited research related to decision-making about tracheostomy for CMC.
The Institute of Medicine and the 2010 Affordable Care Act support shared decision making in clinical practice as a means to improve the quality of care.6,7 An ideal decision-making process involves information sharing between healthcare providers and caregivers both about medical and personal preferences.8 There are gaps in decision science in pediatrics.9 Caregivers need information from clinicians to help them make decisions about LST, but their information needs are largely unmet.10, 11 There are studies about decision-making about gastrostomy tubes in children.12 Because the implications of LSTs on children and families vary by the type of treatment, information about gastrostomy decision-making cannot be extrapolated to decisionmaking about tracheostomy.
Studies on caregivers’ experiences about tracheostomy for children are limited;13 their perspectives about tracheostomy decision-making are lacking. The objective of this paper is to describe caregiver perceptions about their decision to pursue tracheostomy, in particular about satisfaction with their decision, for CMC living at home.
METHODS
We report one aspect of a larger qualitative study to understand healthcare providers’ roles in the tracheostomy decision-making process for CMC. The study included interviews of caregivers of CMC about their perspectives on decision-making about tracheostomy for their children, and focus groups of healthcare providers about their perspectives about healthcare providers’ roles in the tracheostomy decision-making process. The study was conducted from 2013 to 2015 at Brenner Children’s Hospital (BCH), a tertiary care children’s hospital of the Wake Forest Baptist Health system in western North Carolina. BCH has 160 pediatric beds and ~5,000 hospitalizations/ year. Wake Forest Health Sciences Institutional Review Board approved the study. Caregiver interviews focused on all aspects of the decision-making process including caregiver experience, clinician communication, resources used, decisional satisfaction, and religiosity and spirituality (Appendix; available at www.jpeds.com). This paper uses data from the caregiver interviews only, and is limited to caregiver perceptions about their decisions to pursue tracheostomy for CMC, including decisional satisfaction. Methodological details are provided in the Consolidated Reporting of Qualitative Studies (COREQ) Checklist14 (Supplement; available at www.jpeds.com).
Eligibility:
Caregivers of CMC who had a tracheostomy were participants in the study. Children were eligible for the study if they: (1) were <18 years, (2) had a chronic condition, defined as health condition that had lasted or was expected to last ≥ 12 months, (3) had a tracheostomy with or without chronic mechanical ventilation, (4) had the tracheostomy performed ≤5 years prior to the interview, (5) were current patients at BCH or patients at the time of death, and (6) were not institutionalized. Children who had a tracheostomy, but were subsequently decannulated or deceased were eligible as long as the other criteria were met. Caregivers were eligible if they were8 years or older, primarily English- or Spanish-speaking, and the primary caregiver most knowledgeable about the child’s condition and services. Additional caregivers participated in interviews as allowed by the primary caregiver. Bereaved caregivers were included except within 6 months of the child’s death in order to not interfere with the bereavement process.
Recruitment:
Based on the time and resources available to us, we planned to conduct at least 40 interviews to fully capture the decision-making experience of caregivers of CMC in at least 2 ethnic groups. Children were identified from 3 sources: (1) the hospital’s administrative database using procedure codes for tracheostomy (codes 31600 and 31601), (2) a list maintained by the otorhinolaryngology department of children who received tracheostomy, and (3) a list of children referred to the Pediatric Enhanced Care Program (PECP), the pediatric palliative care/complex care program of BCH. A preliminary list of 140 children was generated from these sources. As children received tracheostomies, their names were added to the list. The nurse coordinator of PECP reviewed the list and identified CMC for recruitment to the study. The process of recruiting participants from this list is described in the Figure. Caregivers of children eligible for the study were recruited by the research associate; Spanish-speaking caregivers were recruited by the bilingual social worker of the palliative/complex care program. Of the 140 children, 73 were ineligible for various reasons, primarily due to having had tracheostomy > 5 years. Eighteen were deferred and 49 were eligible. Of those eligible, 8 refused and 41 were enrolled.
Data Collection and Management
Child-level data including diagnosis, date of tracheostomy, birth and death, and insurance type were abstracted from medical records.
Qualitative data were obtained from 41 interviews (35 English and 6 Spanish) of 56 caregivers conducted between December 2013 and November 2014. An interview guide was developed by the study team, based on a conceptual framework informed by existing literature, to elicit information about the tracheostomy decision-making process (Appendix A). In order to capture emerging concepts, the guide was revised twice as interviews progressed; the question about decisional satisfaction was not revised in subsequent versions. In-depth semi-structured interviews were conducted by trained interviewers at locations and times of caregivers’ choosing. English interviews were conducted by the research associate, and Spanish interviews by a social worker. At the time of the interviews, surveys eliciting demographic information were administered. A $30 incentive was provided to participants for each interview. Interviews were audio-recorded, and transcribed verbatim by a professional transcriptionist. Spanish interviews were transcribed first, and then translated into English by a professional translator.
Statistical Analyses
A codebook was developed inductively and revised for accuracy as coding progressed. In a team setting, each code was defined in how it would be applied to text. ATLAS.ti (v.7) software was used for coding transcripts.15 All 4 investigators coded the first 2 transcripts as a group to ensure the consistent understanding and application of codes and their meanings. For the remaining 39 transcripts, 2 investigators independently coded each transcript, then compared them and reconciled coding differences to arrive at a consensus; a clinician (SN/AG) - social scientist (NK/SG) pair coded 33 transcripts to balance academic perspectives on the interviews. As codes and themes emerged during the active coding process, early-coded transcripts were reviewed near the end of the process and re-coded as necessary to ensure consistency of code applications. The transcript excerpts for each code were summarized by an investigator and verified by a second investigator. Discrepancies in interpretation were discussed and noted. We used thematic content analysis.16 This paper focuses only on recurrent themes that emerged related to caregivers’ decisional satisfaction about tracheostomy. Themes were identified by their prevalence and salience in the data. No new themes emerged after 10 interviews. Because this paper is part of a larger study, data collection was continued beyond theme saturation.
RESULTS
Characteristics of children and the caregivers are described in Table 1. The median period of time children had the tracheostomy was 1.5 years (range 2 months to 5 years). The sample included children with a wide range of diagnoses, age at tracheostomy, and household incomes. The median length of interviews was 66 minutes (range: 35 to 175 minutes). Of the 41 interviews, 33 were conducted at children’s homes, 5 in the hospital, 1 in caregiver’s office, and 2 in public places. Themes related to caregivers’ perspectives about tracheostomy decision are presented in Table 2.
Table 1.
Participant Characteristics
| Characteristic | Median (Range) or Number |
|---|---|
| Children whose caregivers participated in interviews [n=41] | |
| Age, median (range)* | 2.5 years (5 months – 18 years) |
| Boys | 21 |
| Girls | 20 |
| White | 29 |
| Black | 10 |
| Multiracial | 2 |
| Hispanic | 7 |
| Health insurance | |
| Medicaid | 31 |
| Private | 7 |
| Both | 3 |
| Annual household income† | |
| < $20,000 | 17 |
| $20,001 to $40,000 | 12 |
| $40,001 to $80,000 | 11 |
| Missing | 1 |
| Primary diagnostic categories | |
| Prematurity | 6 |
| Anoxic brain injury | 5 |
| Myopathy, muscular dystrophy | 7 |
| Neurological malformations | 4 |
| Lung or heart defects | 4 |
| Genetic conditions | 8 |
| Malignancy | 1 |
| Other | 6 |
| Age at tracheostomy, median (range) | 10 months (4 days – 17 years) |
| Duration of tracheostomy, median (range)§ | 1.5 years (2 months – 5 years) |
| Tracheostomy only | 25 |
| Tracheostomy and chronic mechanical ventilation | 16 |
| Decanulated prior to interview | 8 |
| Decanulated and 2nd tracheostomy | 1 |
| Died prior to interview | 3 |
| Caregivers who participated in 41 interviews [n=56] | |
| Age, median (range) | 36 years (19 years – 53 years) |
| Relationship to the child | |
| Mother | 38 |
| Father | 13 |
| Grandmother | 3 |
| Grandfather | 1 |
| Grandmother figure | 1 |
| Education† | |
| < high school | 9 |
| High school | 16 |
| Some college | 11 |
| College | 17 |
| Missing | 3 |
date of birth to date of interview/ date of death
date of tracheostomy to date of interview/ date of decanulation/ date of death
includes missing values
Table 2.
Caregiver Decisions about Tracheostomy: Themes with Illustrative Quotes
| Theme 1: Decisions motivated by child’s survival and provision of home care |
| Survival: |
| “To me it’s a miracle really. Because without that [tracheostomy], he wouldn’t be here. [D03] |
| “It was the right decision… I know he would have never made it without it.” [D14] |
| “I do feel like it has helped her to still be here today. I feel like if I wouldn’t have made that decision, I don’t think that she would.” [D18] |
| “I always had my what-ifs, but, I pretty much know if she wouldn’t have got it she would have passed away.”[D23] |
| Provision of home care: |
| “That was what we had to do to get her out of that hospital…” [D22S] |
| “If it weren’t for that, he would not have been able to get out from the [hospital].” [D38S] |
| Theme 2. Benefits after tracheostomies |
| Respiratory |
| “She’s breathing a lot better and [getting] more oxygen. She’s got more energy. She’s not having to work, gasp for air…she is just playful, starting to play with rattles which she wasn’t doing before.” [D05] |
| Growth |
| “She is growing, she was little bitty, she wasn’t growing, she’s growing, she has hair, her fingernails are growing, she’s laughing, smiling, she’s staying awake.” [D04] |
| Sleep |
| “He is calmer, he sleeps a lot. The nurses say that he wakes up at night, but he goes back to sleep. Perhaps before it was just too much work to breathe and because of that he was uncomfortable and he was irritated all the time… [Now], he can sleep well and peacefully. He can breathe better.” [D40] |
| Quality of life |
| “If you dealt with what I dealt with - his lungs collapsing, him being on the CPAP, him just not being happy… When we got the trach, he was so much happier.”[D20] |
| Theme 3: Negative effects of tracheostomy |
| Respiratory |
| “The first three days that he was home, and then… he had some issues with a lot of secretions and a plug and him getting sick which turned him right back to the NICU.” [D3] |
| Eating |
| “He was a very good eater before the trach… now we’re on pureed diet, he wasn’t like that before the trach… I think the trach plays a part of it, maybe not a major role, but some part of that.” [D27] |
| Voice |
| “… he wasn’t making any sounds. See, before he would cry hysterically. After that, we could not hear him cry. We could tell he was crying because, you know, he would make that face…And that was the first time that I broke down after the surgery…They didn’t really specify or explain that trach babies don’t make sound.” [D21] |
| Theme 4: Difficulties of providing medical care at home |
| “…she got a plug and we were tryin’ to change it, and when we changed it, she was coughing stuff out of her stoma, and we could not get the trach back in. So we had to call 911 and they had to calm us down over the phone, and she was turnin’ really red, and I was crying.” [D07] |
| “I guess how difficult it would be to carry everything that she would possibly need. I think they should tell everybody what you’re going to need. You’re going to need a suction machine, you’re going to have a humidifier 90% of the time. I think they should tell you what you are going to have to have and a little bit more of the bad and not just the good.” [D23] |
| Theme 5: Decisional satisfaction |
| “The only advice I’d give to other parents is the trach is a really good thing, it really is. It’s the best decision we could have made. I mean out of everything, we love the trach. Never thought we’d love a trach, but we love a trach, don’t we?” [asks child, who is physically unable to respond] [D11] |
| “Having a child with a trach is definitely scary, but the decision whether your child actually needs a trach, it’s a reward, kind of. It sounds crazy because you have that hole in your neck, but being able to see [child] breathe normally, seeing her being able to move out of the hospital bed and me being able to pick my baby up from the bed, that was a decision that - I mean I knew I had made the right decision.” [D13] |
| “Well, we feel that in the whole bad situation, it was one of the decisions that was the most viable. The most appropriate one. The correct one. We don’t wish he had a trach, we don’t wish it was there, you know? But it was the most appropriate.” [D38] |
We found that caregivers often chose the tracheostomy procedure for their children because extending the lives of their children and being able to care for them at home were important.
At the time of decision-making, tracheostomy was often the only option presented to them to guarantee life and delay death indefinitely. Children’s survival was the most important reason for caregivers pursuing tracheostomy for their children.
Some caregivers also mentioned that a tracheostomy enabled them to take their child home from the hospital. Their children were intubated for long periods of time and did not tolerate repeated attempts at extubation. Tracheostomy was offered as an option by clinicians in order to be able to discharge children home from the hospital and as an alternative to prolonged hospitalization. Caregivers acknowledged that without the tracheostomy their children would not have been able to be cared for at home.
Caregivers noticed many clinical benefits to the health and well-being of their children after tracheostomies
Tracheostomy resulted in many clinical benefits for children. Several caregivers recalled that prior to the tracheostomy, their children had severe breathing difficulty and did not sleep well; some children had such severe respiratory symptoms that caregivers perceived them to be suffering and were scared that their children would die when asleep. Tracheostomy made it easier for children to breathe without “struggling”.
In addition to benefits with respiratory symptoms, after tracheostomy caregivers noted that their children slept better, were more alert, had improvement in skin color, activity, energy level, and physical growth (i.e. weight gain, hair and nail growth), and made developmental progress. Caregivers reported their children to be less irritable, calmer, more comfortable, and happier after tracheostomy.
Caregivers also found it easier to manage respiratory problems post-tracheostomy, especially clearing secretions by suctioning the tracheostomy tube. Some mentioned that their children had fewer illnesses, handled respiratory infections better, and had fewer and/or less lengthy hospitalizations than before tracheostomy. Many parents expressed their perceptions that the presence of the tracheostomy did not bother the child or affect his/her functioning. Because of the benefits to the well-being of children, several mentioned that their children’s “quality of life” improved after tracheostomy.
Other benefits described by caregivers included their ability to hold their children, which they could not do while they were hospitalized and intubated, and see their faces fully because they no longer needed breathing apparatus that obscured the faces. In addition to specific benefits, several caregivers reported that tracheostomy generally “helped a lot” or “benefited the child”.
Caregivers addressed negative effects of tracheostomies, including actual and feared changes in their children’s health.
Mucus plugs and excessive secretions were reported. The child or sibling pulling out the tracheostomy tube was feared and/or experienced by several caregivers. A few caregivers explained that pre-surgery their children could eat by mouth, but after the tracheostomy this was more difficult or not possible. Caregivers were worried about the increased risk of respiratory infection after tracheostomy, but only very few mentioned that their children had repeated infections.
Immediately after surgery, caregivers admitted being scared of the tracheostomy hole (stoma) and the unattractive appearance of the tracheostomy; they were upset when they saw the tracheostomy tubes in their children for the first time and could not hear their children’s voices. Caregivers got used to the tracheostomy and their fears subsided over time. Although caregivers reported that clinicians prepared them about the risks of tracheostomy, some mentioned that they were not prepared for all the negative effects, especially about the loss of voice after tracheostomy.
Caregiving for children with tracheostomies at home is complex and difficult for families.
Caregivers had to learn many skills related to tracheostomy care – equipment care, tracheostomy ties, suctioning, tracheostomy change, and clinical problem-solving (e.g. mucus plugs, emergency tracheostomy change, etc.). Caregivers were worried about the consequences of poor care (e.g. death, infection, hospitalization) and worked very hard to ensure good quality care for their children. They were scared, overwhelmed, and unsure about their ability to perform these duties initially. The fear of not being able to replace the tracheostomy tube if the tube came out was a cause of caregiver stress. Caregivers acknowledged that their skills improved over time, but some were nervous to perform certain tasks (eg, tracheostomy tube change) even after several years.
Caregivers also described the logistical difficulties of taking children with tracheostomy outside their homes and providing medical care in public places (e.g. suctioning when in a restaurant). Additionally, caring for children with a tracheostomy in the home compromised caregiver privacy, personal work schedules, and the ability to effectively care for their other children. Caregivers reported that they received very little external informal support (e.g. from family) for caregiving. They felt that they were prepared by clinicians in the technical care of children, but were not fully prepared about the difficulty of home care and the marked change in home life.
Overall, caregivers were satisfied with their decisions to pursue tracheostomy for their children.
In 38 interviews (32 out of 35 English and all 6 Spanish) caregivers explicitly expressed satisfaction with their decision to pursue tracheostomy. In describing their decisions they stated tracheostomy to be the “right decision”, “best decision ever made”, and they “don’t regret it”, “will do it again”, are “happy with the choice”, are “grateful and thankful for tracheostomy”, and “would recommend tracheostomy to others”. In 3 interviews, where decisional satisfaction was not explicitly mentioned, the benefits of tracheostomy in enabling/improving children’s survival were described. One of these caregivers mentioned that tracheostomy helped the child, but did not “fix all the problems”. In none of the interviews did caregivers express clear regrets about their decision. Decisional satisfaction did not vary based on child’s age, duration of tracheostomy, survival, underlying condition, being on chronic mechanical ventilation, or subsequent decannulation.
Although decisional satisfaction was high, caregivers acknowledged the many difficulties with tracheostomy. They mentioned that parents should ask doctors about the benefits of tracheostomy and pursue tracheostomy only if absolutely needed.
DISCUSSION
This builds on previous studies by reporting caregivers’ decisional satisfaction with tracheostomy for CMC. A major strength of our study is that it includes the perspectives of caregivers of children with heterogeneous health conditions.
An important reason for caregivers’ decision to pursue tracheostomy for their children was that tracheostomy extended children’s lives. Because there were no other options available to ensure children’s survival, caregivers felt that they did not have a choice other than pursuing tracheostomy. This finding is consistent with a prior study in which caregivers report feeling that they did not make a “free choice” because the alternative was to let their child die.17 Patients, surrogate decision-makers for patients, and parents of pediatric patients often view themselves as having no choice because the health of the patient constrains decision-making. A coercive situation like a child’s serious illness can make free choice seem impossible, but in circumstances like these, the parent’s choice, although difficult, is nonetheless autonomous.18 The caregivers in our study regarded their decisions in favor of tracheostomy as the only choices they could make. However, they did not report that they felt coerced by anyone to decide as they did.
Although tracheostomy is chosen with children’s survival in mind, tracheostomy carries a high mortality risk, especially in children with neurological impairment.19 In fact, four children in our study died subsequent to the interviews. In a prior report, caregivers of children using mechanical ventilation at home feared that their child might die at any time.17 Caregivers in our study did not explicitly express this fear about the mortality risk with tracheostomy; nor did they mention that they were informed about this risk. It is not clear how well physicians counsel caregivers about the mortality after tracheostomy.
Caregivers reported the many benefits of tracheostomy. Prior reports on outcomes of children who received tracheostomy are limited to hospitalization, readmissions, survival, decannulation, and level of functioning.20–23 However, patient-centered outcomes, such as the ones shared by caregivers in our study (e.g. improvement in symptoms, and physical and developmental wellbeing), have not, to our knowledge, been reported before in the medical literature. Patientcentered outcomes are important for clinicians to guide caregivers about decision-making regarding tracheostomy. Hence, longitudinal studies are needed to quantify the outcomes described in our study. In addition, clinical outcomes of children who received tracheostomy should be compared with those who received non-invasive ventilation, and those for whom families chose not to pursue tracheostomy.
In our study, caregivers reported that clinicians did not counsel them adequately about the longterm negative consequences of having a tracheostomy. Hebert et al found that physicians, in family conferences about tracheostomy decision-making, discussed the benefits of tracheostomy more often than the risks.24 We found that tracheostomy has ramifications not only for the child, but also for the family. Caregiver burden in caring for children with tracheostomy has been described before.13 In guiding caregivers through tracheostomy decision-making, clinicians should present both the benefits and harms of tracheostomy, and the effect of tracheostomy on the family. Because physicians of any one particular specialty are unlikely to have comprehensive knowledge to guide families about the effect of tracheostomy on the child and the family, an interdisciplinary team approach is needed for the tracheostomy decision-making process.
One of the reasons for caregivers pursuing tracheostomy for their children was that tracheostomy provided the possibility for children to be discharged from the hospital and cared for at home. Most parents desire to have their CMC cared for at home rather than in institutions.25 Tracheostomy is a means to achieving this goal. Many caregivers mentioned, in addition to specific benefits, improvement in the overall quality of life of CMC after tracheostomy. These observations raise the question about the role of palliative tracheostomy for CMC. Tracheostomies are performed in adults for comfort or for short-term clinical goals (i.e. weaning from sedation or discharge from the hospital).26 This is not an approach common in pediatric care. Marshal et al report 3 cases where tracheostomy was performed as a palliative measure in children.27 The role of tracheostomies in improving quality of life of children requires further examination.
Caregivers were satisfied with their decisions to pursue tracheostomy for their children. Low decisional regret has been reported before,28,29 and is considered attributable in part to postdecision cognitive processes that justify decisions by focusing on positive outcomes and minimizing negative outcomes.30 However, because many caregivers reported negative and difficult aspects of tracheostomy, we do not believe that caregivers’ satisfaction was significantly exaggerated. Prior studies have shown that caregivers are satisfied with gastrostomy tube feeding in children.31 However, caregiver perspectives about gastrostomy tubes12 are distinguishable from, and may be less positive on balance than, the perspectives about tracheostomy that we found in our study. This may be, in part, because gastrostomy tubes compromise oral feeding, which has great social and cultural significance for parents, whereas the effect of the tracheostomy on vocalization, which was mentioned by some caregivers in our study, may not be as troubling. This suggests that perspectives about LST vary based on the type of the treatment.
Contrary to our study’s findings, surrogates making decisions for adults needing tracheostomy had high decisional conflict and regret.26 This is probably because of the differences in the value of tracheostomies in these populations. It is also possible that there are inherent differences in parents making decisions for their children versus surrogate decision-making in adults. It will be important to understand the perspectives of children with tracheostomy directly.
We did not include caregivers of CMC with whom decision-making about tracheostomy was discussed, but not pursued. CMC were identified from the preliminary list by the nurse coordinator of PECP; this might have resulted in selection bias. Caregivers’ recall of experiences may have been affected by their memory and hence findings are subject to recall bias. All children in this study had tracheostomy for 5 years or less. Experiences of those who had a tracheostomy for a longer period of time may be different. We did not find any thematic differences based on ethnicity and other demographic attributes. Because decisional satisfaction was high and some categories had a small number of participants, we may not have discerned differences in perceptions between groups. Because of the qualitative nature of the study, the extent of benefits and harms could not be quantified. Because this study excluded children who were institutionalized, the results of this study are not generalizable to children with tracheostomy who are institutionalized. This study is limited to a single institution. Care delivery and experiences may be different in other institutions or regions of the country. Finally, children’s perspectives on having a tracheostomy were not explored in this study.
Caregivers of CMC are generally satisfied with their decisions to pursue tracheostomy for their children, and this seems due, at least in part, to the fact that tracheostomy extended the children’s lives. Tracheostomy has both benefits and harms for CMC. Caring for CMC with tracheostomies has considerable impact on caregivers. Results of this study can be used to improve the pediatric tracheostomy decision-making process.
Figure 1.
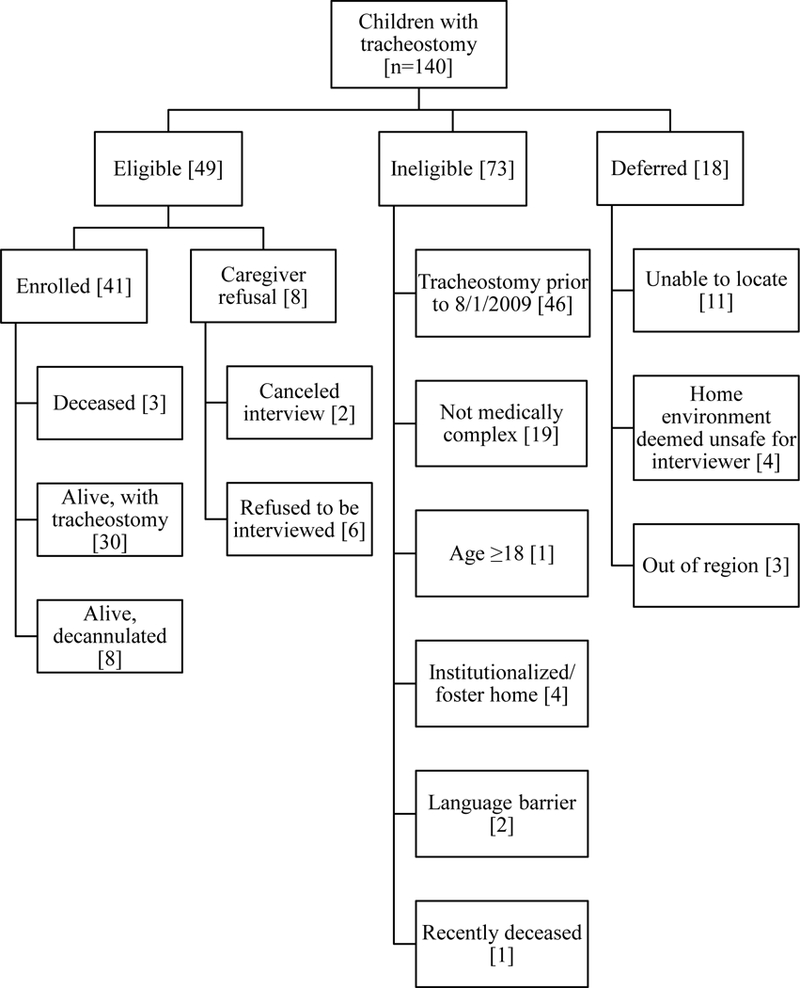
Recruitment of Caregivers Based on the Characteristics of Their Children
Acknowledgement
We thank Ms Aura Rosado MSW, Care Coordinator, Pediatric Enhanced Care Program, Wake Forest School of Medicine (WFSM) for recruiting and interviewing Spanish-speaking caregivers in this study, and for reviewing the code summaries. We thank Ms Vanessa Ortiz, Patient Navigator, Pediatric Enhanced Care Program, WFSM for reviewing the code summaries.
Supported by the National Institute of Nursing Research (R21NR013272 [to S.N.]). The sponsor played no role in study design, collection, analysis or interpretation of the data; in the writing of this manuscript or in the decision to submit the manuscript for publication. The authors declare no conflicts of interest.
Abbreviations:
- CMC
children with medical complexity
- LST
life-sustaining treatments
- BCH
Brenner Children’s Hospital
- PECP
Pediatric Enhanced Care Program
Appendix. Caregiver Interview Questions
Thinking back, tell me about the first time someone talked to you about having this procedure for your child.
-
Who was involved in the initial discussion, and where did the discussion take place?
Probe: How did you feel about the physical setting?
-
What was your reaction to this discussion?
Probe: What were your initial concerns about having this procedure for your child (what was important to you at that time)?
Probe: How did the doctors address these concerns?
Probe: How was the subject matter communicated to you?
Probe: What could have gone better?
-
What other options were you presented? By whom?
Probe: What did you think of those options?
-
Throughout the decision-making experience, who played a key role? Why?
Probe: Who referred you to them?
-
At the time, who, if anyone, did you avoid discussing this with? Why?
Probe: Who were the people who did not support your decision?
The people who advised you – how did they impact your decision?
-
What other resources did you use to help you make the decision? (By resources, I mean things like the internet, books or reading material, other parents)
Probe: How did you find them? (did you seek them or did they come to you?)
Probe: Who recommended them?
What was the most helpful thing or who was the most helpful person to you in making this decision? Why?
What was the least helpful thing or who was the least helpful person to you in making this decision? Why?
-
Now that you know what you do about tracheostomies, how do you feel about the information and advice you received at the time of the decision?
Probe: Did you receive enough information & support? If not, why?
How do you feel now about your decision to give your child a tracheostomy?
-
What are your hopes for your child since the trach was placed?
Probe: What does the future look like for him/her?
-
In your opinion, how can doctors and healthcare professionals best serve families in situations like yours? Please explain.
Probe: communication style
-
What do you know now that nobody told you at the time of the decision?
Probe: What did they tell you to prepare you – what did they say would happen?
-
How did any of your beliefs – your view of the world – play a role in making this decision?
Probe: In other words, how did your beliefs help you decide?
Probe: What – if any – were the spiritual beliefs you considered?
If you have a pastor, in what ways did he/she help you with the decision making process?
Did you talk about your spiritual beliefs with your child’s doctors or nurses when you made this decision – why or why not?
-
Baptist sometimes offers families the services of a chaplain, or pastoral care. Did you use this service? Why/why not?
If yes, tell me how that came about and how it worked out.
How can pastors best serve families in situations like yours?
How would you advise a family who was faced with a decision to give their child a tracheostomy?
Is there anything else you would like to tell me about your decision to give your child a tracheostomy?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SK, Simon TD, et al. Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics. 2011;127:529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feudtner C, Christakis DA, Zimmerman FJ, Muldoon JH, Neff JM, Koepsell TD. Characteristics of deaths occurring in children’s hospitals: Implications for supportive care services. Pediatrics. 2002;109:887–93. [DOI] [PubMed] [Google Scholar]
- 3.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: National trends and implications for supportive care services. Pediatrics. 2001;107:E99. [DOI] [PubMed] [Google Scholar]
- 4.Feudtner C, Villareale NL, Morray B, Sharp V, Hays RM, Neff JM. Technologydependency among patients discharged from a children’s hospital: a retrospective cohort study. BMC Pediatr. 2005;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Com G, Kuo DZ, Bauer ML, Lenker CV, Melguizo-Castro MM, Nick TG, et al. Outcomes of children treated with tracheostomy and positive-pressure ventilation at home. Clin Pediatr (Phila). 2013;52:54–61. [DOI] [PubMed] [Google Scholar]
- 6.Field M, Behrman R. When Children Die: Improving Palliative and End-of-Life Care for Children and their Families Institute of Medicine (US). Committee on Palliative and End-of-Life Care for Children and Their Families.; 2003. [PubMed] [Google Scholar]
- 7.Braddock CH 3rd. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Making. 2010;30:5S–7S. [DOI] [PubMed] [Google Scholar]
- 8.Charles C, Whelan T, Gafni A. What do we mean by partnership in making decisions about treatment? BMJ. 1999;319:780–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyatt KD, List B, Brinkman WB, Prutsky Lopez G, Asi N, Erwin P, et al. Shared Decision Making in Pediatrics: A Systematic Review and Meta-analysis. Acad Pediatr. 2015;15:573–83. [DOI] [PubMed] [Google Scholar]
- 10.Miller JR, Colbert AP, Osberg JS. Ventilator dependency: decision-making, daily functioning and quality of life for patients with Duchenne muscular dystrophy. Dev Med Child Neurol. 1990;32:1078–86. [DOI] [PubMed] [Google Scholar]
- 11.Guerriere DN, McKeever P, Llewellyn-Thomas H, Berall G. Mothers’ decisions about gastrostomy tube insertion in children: factors contributing to uncertainty. Dev Med Child Neurol. 2003;45:470–6. [DOI] [PubMed] [Google Scholar]
- 12.Mahant S, Jovcevska V, Cohen E. Decision-making around gastrostomy-feeding in children with neurologic disabilities. Pediatrics. 2011;127:e1471–81. [DOI] [PubMed] [Google Scholar]
- 13.Flynn AP, Carter B, Bray L, Donne AJ. Parents’ experiences and views of caring for a child with a tracheostomy: A literature review. Int J Pediatr Otorhinolaryngol. 2013;77:1630–4. [DOI] [PubMed] [Google Scholar]
- 14.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57. [DOI] [PubMed] [Google Scholar]
- 15.ATLAS.ti. 7.2 ed. Berlin: Scientific Software Development GmbH; 2013. [Google Scholar]
- 16.Green J, Thorogood N. Qualitative methods for health research: Sage; 2013. [Google Scholar]
- 17.Carnevale FA, Alexander E, Davis M, Rennick J, Troini R. Daily living with distress and enrichment: The moral experience of families with ventilator-assisted children at home. Pediatrics. 2006;117:e48–60. [DOI] [PubMed] [Google Scholar]
- 18.Faden RRB, King TL, N.M.P. A History and Theory of informed Consent. NY: Oxford University Press; 1986. [Google Scholar]
- 19.McPherson ML, Shekerdemian L, Goldsworthy M, Minard CG, Nelson CS, Stein F, et al. A decade of pediatric tracheostomies: Indications, outcomes, and long-term prognosis. Pediatr Pulmonol. 2017;52:946–53. [DOI] [PubMed] [Google Scholar]
- 20.Berry JG, Graham RJ, Roberson DW, Rhein L, Graham DA, Zhou J, et al. Patient characteristics associated with in-hospital mortality in children following tracheotomy. Archives of disease in childhood. 2010;95:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funamura JL, Yuen S, Kawai K, Gergin O, Adil E, Rahbar R, et al. Characterizing mortality in pediatric tracheostomy patients. Laryngoscope. 2017;127:1701–6. [DOI] [PubMed] [Google Scholar]
- 22.Edwards EA, O’Toole M, Wallis C. Sending children home on tracheostomy dependent ventilation: Pitfalls and outcomes. Arch Dis Child. 2004;89:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry JG, Graham DA, Graham RJ, Zhou J, Putney HL, O’Brien JE, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebert LM, Watson AC, Madrigal V, October TW. Discussing Benefits and Risks of Tracheostomy: What Physicians Actually Say. Pediatr Crit Care Med. 2017;18:e592–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk S, Glendinning C. Developing services to support parents caring for a technologydependent child at home. Child: Care, Health & Development. 2003;30. [DOI] [PubMed] [Google Scholar]
- 26.Miller JJ, Morris P, Files DC, Gower E, Young M. Decision conflict and regret among surrogate decision makers in the medical intensive care unit. J Crit Care. 2016;32:79–84. [DOI] [PubMed] [Google Scholar]
- 27.Marshall V, Holt F, Crowe S. Tracheostomy as a Comfort Measure in Children With Life-Limiting Conditions. J Palliat Care. 2017;32:89–91. [DOI] [PubMed] [Google Scholar]
- 28.Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281–92. [DOI] [PubMed] [Google Scholar]
- 29.Mack JW, Cronin AM, Kang TI. Decisional Regret Among Parents of Children With Cancer. Journal of Clinical Oncology. 2016;34:4023–9. [DOI] [PubMed] [Google Scholar]
- 30.Differentiation Svenson O. and Consolidation Theory of Human Decision-Making - a Frame of Reference for the Study of Predecision and Postdecision Processes. Acta Psychol. 1992;80:143–68. [Google Scholar]
- 31.Matuszczak E, Hermanowicz A, Klek S, Komarowska M, Pawlowska D, Zoubek-Wojcik A, et al. Parents’ Perceptions of Gastrostomy Feeding for Children With Neurological Disabilities A Multicenter Study. J Hosp Palliat Nurs. 2014;16:521–5. [Google Scholar]


