Abstract
Objectives
To estimate the prevalence of disabling chronic pain (DCP) in Spanish adults, to analyse its characteristics, to determine its multimorbidity and to identify its associated factors.
Settings
2011 Andalusian Health Survey, a cross-sectional population survey based on face-to-face home interviews.
Participants
6507 people aged 16 years or older and living in Andalusia, Spain.
Outcomes
The response variable was disabling chronic pain. Multivariate multinomial logistic regression models were used to analyse the association of factors with disabling chronic pain. The sample design was considered throughout the statistical analysis.
Results
The prevalence of disabling chronic pain in the Spanish adult population was 11.36% (95% CI 11.23 to 11.49), while that of non-disabling chronic pain was 5.67% (95% CI 5.57 to 5.77). Disabling chronic pain was associated with high multimorbidity (especially in women (51%) and in the elderly (70%) with three or more additional chronic diseases), as well as with disadvantaged social status (such as female gender (OR=2.12), advanced age (OR10-year increase=1.28), unemployment (OR=1.33), manual work (OR=1.26), low income (OR=1.14) and reduced emotional social support (OR=1.04)). Other influential factors were tobacco consumption (OR=1.42), sleeping ≤7 hours (OR=1.2)], environmental or work conditions (OR=1.16) and quality of life (ORmental=1.21, ORphysical=2.37).
Conclusions
The population with disabling chronic pain was associated with multimorbidity, vulnerable social status and an impaired quality of life. In contrast, the population with non-disabling chronic pain showed almost no differences when compared with the population without chronic pain. The association between DCP and mental disorders highlights the need for psychosocial services in the management of chronic pain.
Keywords: chronic pain, disability, multimorbidity, activity restriction, cross-sectional study, quality Of life
Strengths and limitations of this study.
This study provides a comprehensive epidemiological approach to disabling chronic pain.
It includes information on chronic pain and disability, which is not available in other important population health surveys, such as the European Health Interview Survey.
It is based on a large-scale cross-sectional population-based survey, which is a reliable source of information. In addition, special efforts were made to avoid sampling biases.
However, it does not include muscle and joint pain in the lower and upper extremities (except the shoulder) nor various traumatological, postsurgical or neuropathic conditions.
It would have been preferable to construct the chronic pain variable from one simple overall question rather than from other chronic disease variables.
Introduction
Estimations of the prevalence of chronic pain (CP) have varied widely among studies.1–4 It has been estimated to range between 12% and 42% worldwide (in people over 18 years old), between 12% and 30% in Europe5 and between 19% and 30.7% in the USA.2 4 It was reported to be 35% in Canada,1 18.5% in Australia,6 17.5% in Japan,7 35% in Hong Kong,8 42% in Sao Paulo9 and between 12% and 17.25% in Spain.3 5 10
Most population health surveys on CP have considered it as a symptom of different chronic diseases, while others have considered CP as an independent entity and have associated it with various comorbidities.11 12 These studies, on the basis of allostatic load models,13 found that the capacity of individuals to adapt to stress factors can be impaired by the presence of CP and two or more comorbidities, thus increasing health risks.
The impact of CP is greater when it limits activities of daily living (ADLs).2 3 5 14–16 The WHO includes disability-related ADL limitations within the ‘International Classification of Functioning, Disability, and Health’ Model.17 This biopsychosocial model considers disability as a state of impaired functioning associated with disease, disorder, lesion or other health conditions, when it is experienced as a deficiency, a limitation on activity or a restriction to participation in any area of life. There have been numerous studies on disability in different diseases but few on its relationship with CP. These studies found a higher frequency of ADL-limiting CP or disabling CP (DCP) in women and in individuals with a lower socioeconomic level, health-related unemployment, elevated depression indicators14–16 and a higher number of visits to their physician.18 However, questions remain regarding the differences between DCP and non-disabling CP (nDCP) and their effects.
With this background, the objectives of this study were to calculate the prevalence of DCP in Spanish adults through key sociodemographic characteristics, to determine its multimorbidity and to identify associated factors.
Material and methods
Design
The Andalusian Health Survey (EAS (Spanish acronym)),19 the information source, is a population-based and cross-sectional survey that uses face-to-face home interviews. It is designed to evaluate the health of population and their usage of health services in Andalusia, Spain. The study population was adults (≥16 years) living in Andalusia. Those people who were institutionalised (eg, hospitals, nursing home and prison) were excluded from the survey, as well as those with cognitive difficulties as to be interviewed.
A multistage stratified sample design was adopted for our research. The sampling units were municipalities, census tracts, households and individuals. The strata were province (8), size of municipality (5) and season of the year (4). Municipalities and census tracts were selected in proportion to the population size, while households were selected with equal probability by systematic sampling. The interviewees applied quotas for each province as well as quotas for sex–age and the size of municipality within each province. A virtually constant assignation was performed per census tract (7–10 adults), and one adult per household was selected for interview. The information was collected between March of 2011 and February of 2012 (for further details please refer to the health survey report).19
Sample and data collection
One hundred and twelve municipalities and 696 census tracts were selected, and 6507 valid personal face-to-face interviews were conducted at home (P=q=0.5; confidence level=95%; Precision=0.0149; design effect=1.525), with a response rate of 67.9% (the no respondent percentage was due to refusal to participate once the household had been contacted). The average interview time was 28.84 min (SD=6.8, median=30 min).
The effects of non-coverage were minimised by selecting the study population within a sampling framework based on census districts and households. To minimise non-response, the interviews were held 7 days per week between 10:00 and 21:00, and interviewers were trained in both field work and in the study’s methodology. In addition, the survey administration was supervised and followed up, and non-responders were replaced with people of the same sex and age in a randomised manner from the same district. Moreover, we also took measures to minimise information/observation/measurement biases by providing adequate training for interviewers (see above) and by following interviews up either in person or with telephone calls (43.1%). The questionnaire was designed with filters and controls to facilitate verification of its correct completion (100% of questionnaires were reviewed), and the sampling design was considered in the data analyses.
Patient and public involvement
This study did not involve patients and the public.
Variables
The study variable was DCP. This is composed of disability (WHO, 2006) and CP.20 21 The disability definition encompasses impairments, activity limitations and participation restrictions. The question about impairments (problems in body function/structure) was whether a doctor or a nurse had told the interviewees that they suffered from any of a wide list of chronic diseases (box 1). It was asked during home-based face-to-face interviews. Activity limitation and participation restrictions were constructed as population who declared that they were limited in their activity when asked about each of the chronic diseases listed (ie, they were asked about it for each chronic disease). Finally, CP was established according to those individuals who reported a chronic disease that included the word ‘pain’, namely: ‘migraine/headache/chronic cephalalgia/frequent headache’; ‘angina/chest pain’; ‘back pain, neck pain, shoulder pain, waist pain, cervical/low back pain’; or ‘menstrual pain’. The independent variables are also listed in box 1.
Box 1. Study variables.
Disabling chronic pain (DCP; dependent variable): population with chronic pain (CP) who declared being limited in their activity by any of the above-reported chronic pains. The non-disabling CP and non-CP population was also defined with this variable
CP: individuals who declared that a doctor or a nurse had told them that they suffered from one or more of the following Chronic Diseases in the survey that included the word ‘pain’20 21 : ‘migraine/headache/chronic cephalalgia/frequent headache’, ‘angina/chest pain’, ‘back pain, neck pain, shoulder pain, waist pain, cervical/low back pain’ or ‘menstrual pain’.
Demographic and economic characteristics: Sex and age (age groups: 16–44 years; 45–64 years; +65 years), marital status, cohabitation, living alone at home, social class53 educational level, employment status, economic difficulty to make ends meet and total revenues.
Number of the following chronic diseases reported (at home, face-to-face) by the individuals: cancer, diabetes, hypertension, high cholesterol, colitis and chronic intestinal disease/inflammatory bowel disease; stomach ulcer; chronic constipation; chronic lung disease; asthma; cardiac disorder; heart attack; fibromyalgia; chronic skin problems; chronic allergy; anaemia; poor circulation; varicose leg veins; haemorrhoids; stroke; depression or anxiety; other mental problems; hearing loss; cataract; arthritis or rheumatism; osteoporosis; cirrhosis; kidney disease; urinary incontinence; infertility; prostate disorder (men); and thyroid diseases. Chronic diseases in the CP dependent variable were excluded.
Health-related quality of life (physical and mental component; 12-Item Short Form Health Survey (SF-12)).54
Question on self-rated health status in the last 12 months.55
Functional social support: total score and as confidant and affective dimensions.56
Sleep and rest during sleeping hours,
Limitation, disability or physical, sensory or learning disabilities for more than 6 months.
Healthy eating habits as57: 1.5 or more litres of water per day; milk, fruit, vegetables and fish: three or more times per week; bread and cereal: one or more times per week; legume, pasta, rice and potatoes: three or more times per week (without being daily) or less than once per week; meat: two or more times per week (without being daily); sausage: one or two times per week or never/almost never; eggs: one or two times per week. Sweets: less than once a week or never/almost never.
Suspected alcoholism,58 frequency of consumption of alcoholic beverages and tobacco consumption.
Body mass index (BMI) as continuous variable and categorised as: low weight (BMI <18.5 kg/m2); normal weight (18.5 kg/m2≤BMI<25 kg/m2); overweight (25 kg/m2≤BMI<30 kg/m2); and obesity (BMI≥30 kg/m2).59 Both size and weight were measured objectively.
Physical activity in the workplace and physical exercise in free time.
Environmental quality of the area of residence from responses to general self-assessment questions and items on noise, smell, air pollution, industry, green areas, delinquency/insecurity and heavy traffic.
The sum of the scores for these items was calculated and then categorised into tertiles (q33.34=18; q66.66=19). Factor analysis was also performed using these variables, obtaining the following two main factors: bad odours and atmospheric pollution; and safety, noise and green spaces.
Physical work conditions (working population): The sum of the scores for the seven items (Likert scale responses 1–4) was calculated and then categorised into tertiles (q33.34=20; q66.6=24).
Psychosocial level occupational exposure60 (working population), considering two components: (1) psychological demands; and (2) active work and development possibilities, such as influence, skill and time control. For both components, the sum of the scores for the corresponding items (Likert scale responses 1–5) and then categorised into three tertiles (q33.34=10 y q66.66=15, component 1; q33.34=26 y q66.66=34, component 2).
Statistical analysis
Data, for dependent variables and their crossing with independent variables, were reported on estimations based on the sampling design for percentages, means, population totals, 95% CIs, sampling errors, coefficients of variation, corrected typified residuals and p values obtained in the statistical tests (Pearson’s χ2 test corrected with second-order Rao-Scott and Mann-Whitney U tests). Estimations for Spain on CP, DCP and nDCP prevalences, populations and variances were calculated by applying a calibration technique based on marginals and on the χ2 distance. In accordance with the calibration requirements,22 the auxiliary variables selected were sex, age, educational level and employment status. The ‘sampling’ R package23 was used for the sample design and calibration weightings in estimations of DCP prevalence and ‘samplingVarEst’ package24 for its variance estimation.
Factor analysis was performed on environmental quality items (box 1), and multivariate multinomial logistic regression models were used to analyse the association of factors with DCP, nDCP and absence of CP (nCP). A model was initially adjusted using a backwards-stepwise procedure, using sociodemographic variables as control variables along with the remaining secondary variables. Those furthest from significance (at 5%) were successively and manually excluded, verifying at each step that the exclusion did not change the value of the other parameters by >30% of their previous value. Variables were re-entered in the model as confounding variables if a change >30% was observed.25 Variables with missing data for over 3.5% of a subpopulation (eg, working population and population over 65 years old) or treated differently (eg, categorisation or coding) were not included in the multivariate. The effects of age and gender interactions with the remaining independent variables were also verified in the data modelling process, and only those that were statistically significant were considered in the final model. Model assumptions were verified using residuals, model convergence, continuous variable linearity, variations in estimation SE and Nagelkerke R-square values.26 With respect to collinearity, it was checked by studying covariates correlation (rho >0.7) and checking parameter correlations. The association between those included in the model was lower than 0.3.
Simple and stacked bar graphs and OR synthesis graphs were created. We used advanced sampling module of SPSS as well as an approximation of sampling with replacement. This gave the equivalence with probability proportional to size sampling.27 Individual case weight was used to adjust for municipality’s population28 following the method described in the Andalusian Health Survey.19
Significance was considered at 5%, and the sample design was considered throughout the statistical analysis (descriptive, bivariate and multivariate).
Results
The main sociodemographic, economic and daily life habits characteristics of the study population as well as the number (%) missing for each variable are listed in online supplementary data as tables 1 and 2.
Disabling CP: prevalence
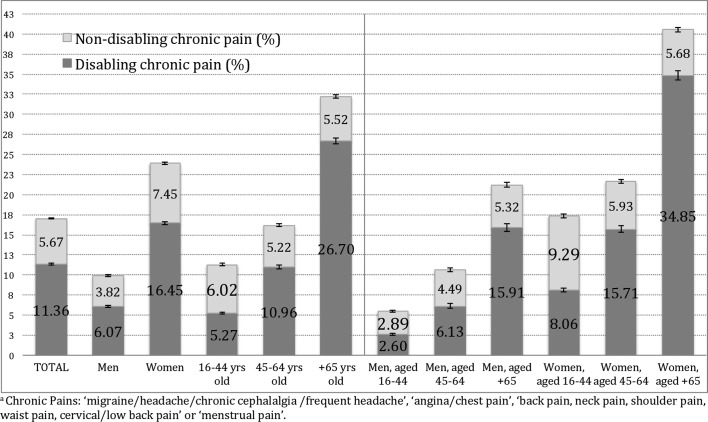
The prevalence of CP in the Spanish adult population was 17.03% (95% CI 16.88 to 17.19), in which 11.36% of that population suffered from DCP (95% CI 11.23 to 11.49; 4 441 556 individuals), while nDCP was reported by 5.67% (95% CI 5.57 to 5.77; 2 178 107 individuals). Of the participants with CP, pain was considered responsible for limitation in some daily life activities by 67% (table 3, supplementary data online). DCP prevalence was threefold higher in women than in men up to the age of 45 years old and twofold higher in older ages. nDCP was significantly more frequent in women versus men up to the age of 45 years old, but there was no significant gender difference in older ages (figure 1). The mean age in the population with DCP was 58.5 years (95% CI 57.2 to 59.8), which is significantly higher than in the population with nDCP and nCP (45.3 and 43.7 years, respectively; p<0.001]).
Figure 1.
Spanish prevalence of disabling chronic pain and non-disabling chronic paina by sex and age groups.
bmjopen-2017-020913supp003.pdf (77.2KB, pdf)
Disabling CP: characteristics
The prevalence of DCP was significantly higher (p<0.001) among the following: those who lived alone (19.5%), widows/widowers (29.6%) and unskilled workers (15.1%); those who were illiterate (28.8%); those literate but with no schooling (24.9%); those who had only received primary schooling (15%); those reporting difficulties in reaching the end of the month (14.1%); and those with a net household income <1000€/month (17%). However, nDCP was not significantly associated with any one of these characteristics.
A significantly higher likelihood of DCP (adjusted for age and sex) was found in those belonging to manual labour social classes (ORmanual=1.26), those with a lower schooling level (ORIlliterate or literate but with no schooling=1.61; ORPrimary schooling=1.57), those who were unemployed but had worked previously versus those in employment (OR=1.33) and the residents of more rural areas (OR=1.28; table 1).
Table 1.
Sociodemographic characteristics of disabling chronic pain and non-disabling chronic pain*†
| Outcomes | Independent variables | Categories | P values | OR | 95% CI | |
| Minimum | Maximum | |||||
| Disabling chronic pain (reference category: no chronic pain) |
Social class (p=0.68) |
I. Manager with 10<=salaried staff | 0.196 | 0.704 | 0.413 | 1.199 |
| II. Manager with 10>salaried staff | 0.231 | 0.776 | 0.512 | 1.176 | ||
| IIIa. Administrative staff | 0.215 | 0.806 | 0.573 | 1.134 | ||
| IIIb. Self-employed | 0.187 | 0.73 | 0.458 | 1.165 | ||
| IIIc. Manual work supervisor | 0.673 | 0.839 | 0.37 | 1.9 | ||
| IVa. Qualified manual worker | 0.603 | 0.929 | 0.704 | 1.226 | ||
| IVb. Manual worker | 0.836 | 0.973 | 0.748 | 1.265 | ||
| V. Unskilled worker | – | 1 | – | – | ||
| Social class (short version) (p=0.107) |
Non-manual (I–III) | – | 1 | |||
| Manual (IV and V) | 0.034 | 1.259 | 1.017 | 1.56 | ||
| Level of education (p=0.056) |
Illiterate/no formal education | 0.014 | 1.615 | 1.104 | 2.364 | |
| Primary education | 0.008 | 1.57 | 1.127 | 2.187 | ||
| Lower secondary/first-cycle vocational training | 0.139 | 1.366 | 0.903 | 2.066 | ||
| Upper secondary/second cycle vocational training | 0.378 | 1.197 | 0.802 | 1.786 | ||
| University education | – | 1 | – | – | ||
| Employment situation (p<0.001) |
Unemployed but previously worked | 0.047 | 1.327 | 1.004 | 1.754 | |
| Seeking first job or student | 0.031 | 0.444 | 0.213 | 0.929 | ||
| Retired (previously employed) | <0.001 | 1.86 | 1.347 | 2.567 | ||
| Home keeper | 0.214 | 1.199 | 0.9 | 1.598 | ||
| Handicap/permanent disability | <0.001 | 5.976 | 3.897 | 9.166 | ||
| Employed | – | 1 | – | – | ||
| Net monthly household income (p=0.024) |
−999€ | 0.61 | 1.14 | 0.688 | 1.889 | |
| 1000–1499 € | 0.617 | 1.136 | 0.689 | 1.873 | ||
| 1500–2499€ | 0.448 | 0.817 | 0.484 | 1.378 | ||
| +2500€ | 1 | |||||
| Rurality index (p=0.05) |
Rurality (continuous) | 0.02 | 1.28 | 1.04 | 1.576 | |
| Non-disabling chronic pain (reference category: no chronic pain) | Social class (p=0.68) |
I. Manager with 10<salaried staff | 0.321 | 0.68 | 0.317 | 1.458 |
| II. Manager with 10>salaried staff | 0.12 | 1.49 | 0.902 | 2.463 | ||
| IIIa. Administrative staff | 0.454 | 1.188 | 0.756 | 1.868 | ||
| IIIb. Self-employed | 0.977 | 0.991 | 0.52 | 1.886 | ||
| IIIc. Manual work supervisor | 0.232 | 0.295 | 0.04 | 2.187 | ||
| IVa. Qualified manual worker | 0.461 | 1.163 | 0.778 | 1.739 | ||
| IVb. Manual worker | 0.34 | 1.205 | 0.821 | 1.77 | ||
| V. Unskilled worker | – | 1 | – | – | ||
| Social class (short version) (p=0.107) |
Non-manual (I–III) | – | 1 | |||
| Manual (IV and V) | 0.882 | 1.021 | 0.773 | 1.35 | ||
| Level of education (p=0.056) |
Illiterate/No formal education | 0.347 | 1.268 | 0.773 | 2.081 | |
| Primary schooling | 0.97 | 0.993 | 0.68 | 1.45 | ||
| Lower secondary/first cycle vocational training | 0.21 | 1.329 | 0.852 | 2.074 | ||
| Upper secondary/second cycle vocational training | 0.482 | 0.845 | 0.528 | 1.351 | ||
| University education | – | 1 | – | – | ||
| Employment situation (p<0.001) |
Unemployed but previously worked | 0.943 | 0.988 | 0.714 | 1.368 | |
| Seeking first job or student | 0.552 | 0.848 | 0.492 | 1.46 | ||
| Retired (previously employed) | 0.942 | 1.017 | 0.649 | 1.592 | ||
| Home keeper | 0.121 | 0.737 | 0.502 | 1.084 | ||
| Handicap/permanent disability | 0.217 | 1.587 | 0.763 | 3.303 | ||
| Employed | – | 1 | – | – | ||
| Net monthly household income (p=0.024) |
−999€ | 0.606 | 0.84 | 0.432 | 1.631 | |
| 1000–1499 € | 0.449 | 1.28 | 0.676 | 2.424 | ||
| 1500–2499€ | 0.693 | 1.141 | 0.593 | 2.195 | ||
| +2500€ | – | 1 | – | – | ||
| Rurality index (p=0.05) |
Rurality (continuous) | 0.309 | 1.158 | 0.872 | 1.538 | |
*Chronic pains: ‘migraine/headache/chronic cephalalgia/frequent headache’, ‘angina/chest pain’ and ‘back pain, neck pain, shoulder pain, waist pain, cervical/low back pain’.
†Variables with p<0.2 are included in the multinomial logistic regression models (except for social class). Variables not included in the multivariate: missing data for over 3.5% of a subpopulation (eg, working population, population over 65 years old) or treated differently (eg, categorisation or coding). All models were adjusted for age and sex. Significance level=0.05. Interactions performed: sex * age, sex * independent variable analysed and age * independent variable analysed, showing the results that were statistically significant and did not affect the model convergence.
Disabling CP: multimorbidity
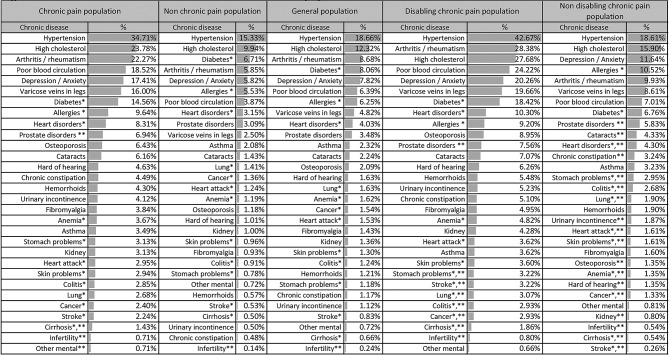
The 10 most prevalent chronic diseases were the same in the different subpopulations, with the exception of prostate disorder, which was replaced by osteoporosis in the DCP subpopulation. The prevalence of a chronic disease, regardless of which, was around twofold or threefold higher in those with DCP than in those with nDCP, and threefold or fourfold higher in those without CP (figure 2). Conversely, DCP prevalence was around threefold or even fourfold higher among those with a chronic disease, regardless of which, while this difference was not seen in the prevalence of nDCP.
Figure 2.
Prevalence of chronic diseasesa in the studied subpopulations.b
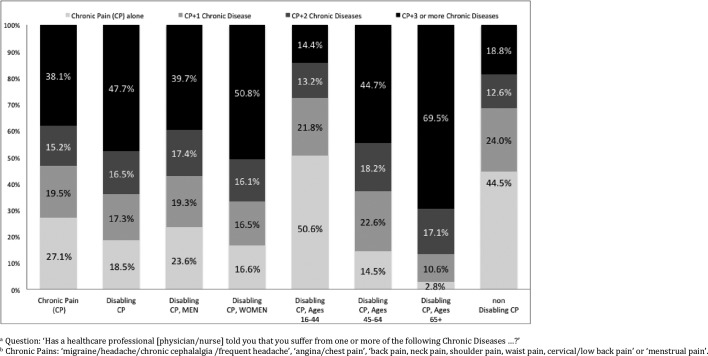
At least one chronic disease was present in 81.5% of the population with DCP versus 40.3% of the population without CP and 55.5% of the population with nDCP (p<0.001; figure 3). At least three other chronic diseases were reported in 47.7% of the population with DCP versus 18.8% of the population with nDCP. There was a strong tendency for the frequency of multimorbidity to be higher in women (versus men) among those with DCP (83.4% and 76.4%; p=0.054) but not among those with nDCP (p=0.45). The mean number of chronic diseases in women with DCP was significantly higher than in men with DCP (3.09, 95% CI 2.85 to 3.33 vs 2.32, 95% CI 2.03 to 2.62) and threefold higher than in women without CP (0.97, 95% CI 0.92 to1.03).
Figure 3.
Multimorbiditya according to subpopulations with chronic painb.
DCP prevalence was fivefold higher among those with other chronic diseases than among those without (20.4% vs 3.9%, respectively, p<0.001). A similar result for gender and for age group was observed. However, the differences of nDCP prevalence among those with and without chronic diseases were much smaller, with the exception of the youngest age group (table 3, supplementary data online).
Disabling CP: associated factors
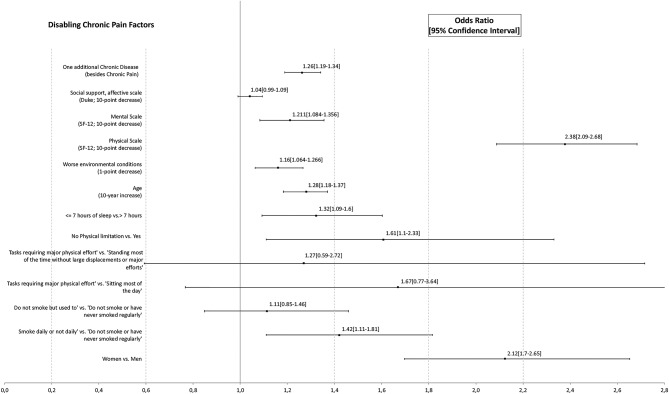
The final multivariate model for factors associated with DCP (figures 4 and 5) used valid data from 96.65% of the study sample (n=6289), and it was highly significant (p<0.001; R2 Nagelkerke=0.27).
Figure 4.
Factors associated with disabling chronic paina,b.
Figure 5.
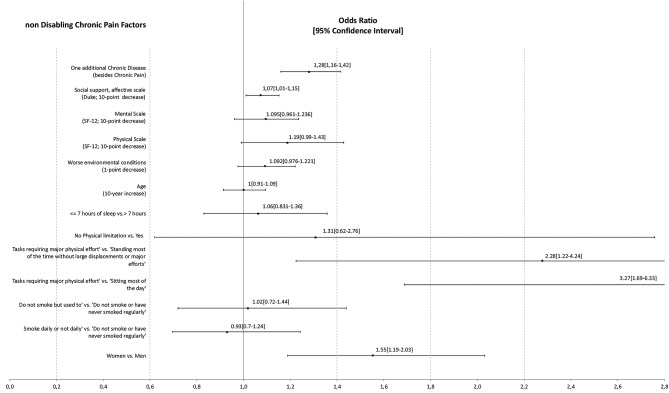
Factors associated with non-disabling chronic paina,b.
The likelihood of DCP versus nCP was significantly higher in women (OR=2.12, p<0.001), individuals sleeping ≤7 hours (OR=1.32, p=0.004), those with some physical limitation (OR=1.61, p=0.012) and smokers (OR=1.42; p=0.005) but not significantly higher in ex-smokers or in those individuals who did ‘heavy work, tasks requiring great physical effort’. A higher likelihood of DCP was also observed in older age (OR10yrs=1.28; p<0.001), the presence of other chronic diseases (OR1chronicdisease=1.26, p<0.001), worse environmental conditions (OR1point=1.16; p=0.001), worse physical (OR10points=2.38, p<0.001) or mental (OR10points=1.21, p=0.001) quality of life and (although this did not reach significance) lower emotional social support (OR10points=1.041, p=0.096).
The probability of non-disabling CP was significantly higher in: women (OR=1.55, p=0.001); individuals with ‘heavy work, tasks requiring great physical effort’ versus those ‘standing most of the time without much walking or major effort’ (OR=2.28, p<0.001) and those ‘sitting during most of the day’ (OR=3.27, p=0.009); those with less emotional social support (OR10points=1.073, p=0.023); and those with other chronic diseases (OR1CD=1.28, p<0.001). In contrast, the likelihood of non-disabling CP was not significantly associated with the physical or mental quality of life, age, environmental conditions, hours of sleep, physical limitations or smoking.
Discussion
Our results show that important differences were observed between populations with disabling and non-disabling chronic pain. The failure to distinguish correctly between DCP and nDCP or their related risk factors may have major negative repercussions on the design of interventions to prevent and treat pain as well as on estimates of the size of this public health problem. The present findings are therefore highly relevant for healthcare policy makers and professionals.
DCP definition and prevalence
The item for measuring activity limitation was also used to measure the participation restrictions (problems in involvement in life situations) in the definition of disability.17 Although other authors29 use the 8-Item Short Form Health Survey (SF-8) scale item on interference with social activities due to physical health or emotional problems, we decided not to consider that item because it is not specific to chronic pain. In fact, it did not obtain high concordance with our already constructed DCP (kappa=0.34). In addition, people interviewed were specifically asked if they were limited in their activity by the reported chronic pains (box 1). So the disability is due to the chronic pain, not to other medical condition. The basis of the definition of CP in this study is the medical or healthcare professionals’ diagnosis (reports of more than 3 months suffering the chronic disease that included the word ‘pain’).20 21 However, survey limitations detected in this study include the need to add muscle and joint pain in the lower and upper extremities (except shoulder) and various traumatological, postsurgical and neuropathic conditions. It would also be preferable to gather direct data on CP with a simple overall question12 and to avoid its construction based on other chronic diseases. By doing this, the possibility of overestimating its prevalence would be reduced. Gathering data on the time since CP onset, using 6 months as the criterion for chronicity,5 is also recommended. Finally, our study did not gather information to analyse neuropathic, nociceptive or dysfunctional pain because this is not essential information as these entities are considered as different points on the same continuum.30
DCP, as observed in our study, is a highly relevant public health problem, as it affects two-thirds of the population with CP. Although there are very few population-based studies on DCP, the Spanish prevalence provided by our study (11.36%) is similar to findings in Canada (range: 11.4%–13.3%)31 and higher than those reported in Germany (7.4%).29 This health problem is especially relevant in women6 32 and individuals aged over 65 years.32 The greatest gender difference observed in our study was in the lower age groups.6 In addition, nDCP could lead to DCP in time, especially in middle age and over. This can be seen in figure 1 where nDCP prevalence is quite similar in the highest age groups, regardless of gender, while DCP prevalence presents much higher differences. Moreover, as showed in online supplementary table 4, the change over time of nDCP into DCP could be much faster among people with other chronic diseases.
One of the largest differences between the DCP and nDCP populations is the mean age, which was 13 years older in the DCP population. Moreover, there was a negligible difference in mean age between those with nDCP and those without CP. Those age differences remained when controlled by the other independent variables. Thus, a much higher likelihood of DCP (vs no CP) in older age was observed, while the likelihood of nDCP (vs no CP) was not significantly associated with age. Furthermore, according to our definition of DCP, in the DCP group, the disability would be pain provoked, and the likelihood of that disability would increase by 28% with every 10 years of age.
Multimorbidity
The presence of other chronic diseases was reported by half of the population without CP, by almost three-quarters of the population with CP and by over four-fifths of the population with DCP. Among individuals with DCP, multimorbidity was much more frequent in women.33 This gender difference grew with increased age in the DCP population, but again, these differences were not observed in the population with non-disabling CP. In general, the prevalence of DCP is fivefold higher among those with other chronic diseases than among those without (figure 2). The gender difference in the prevalence of DCP was even greater among those with other chronic diseases. According to allostatic load models,12 CP is more disabling in patients with a larger number of chronic diseases, thus increasing their health risk.11 12
The prevalence of diseases such as fibromyalgia, arthritis or rheumatism/osteoporosis was significantly higher in women with DCP when compared with women with nDCP population or women without CP, while the prevalence of those diseases in men is too low to observe significant differences. In general terms, the prevalence of those chronic diseases between the nDCP population and the population without CP does not differ significantly. However, results obtained in the DCP population showed much higher prevalence (figure 2). It is not clear, due to the variability within those chronic diseases, that they always result in pain.34–39
The prevalence of arterial hypertension in the DCP population was more than double that in the nDCP or nCP populations. The mechanisms underlying the association between CP and hypertension have not been fully elucidated, and the allostatic factors involved remain under discussion.40 41 The population with depression or anxiety showed a prevalence of DCP that was threefold higher than in the population without, signifying that there is an increase in disability when CP is associated with depression or anxiety.6 14 15 42–44 We consider these results with caution for two reasons. First, from a neurological point of view, pain and depression interact in a complex relationship of situational and physiological connections that is not yet fully understood.45 Second, depression and anxiety were measured together in our study through the same variable. Despite this, the association between DCP and these mental disorders highlights the need for psychosocial services in chronic pain management.29
DCP-associated factors
Our study showed that DCP was also associated with having only primary education, being unemployed after having worked previously, unqualified/unskilled employment, low income, low functional social support, poor health habits, impaired quality of life, worse environmental or work conditions and rural life. Further research is necessary on the interaction of lower educational attainment, employment status and type of work. A statistical significant association was found between worse health-related quality of life and DCP but not with nDCP. Both components of functional and social support, which are considered to play an important role in helping sufferers cope with their pain,46 47 were significantly lower in the population with DCP, whereas the result was significantly lower for those with nDCP only in the affective component. These results go further than those provided by other studies.6 48 49
Survey features
Beyond the intrinsic limitations of cross sectional studies such as poor recall or overemphasis on recent events,50 the strength of this study is that it is based on a large-scale population-based survey. Its complex design (multistage stratified sample), large sample size (6.507 individuals), very good response rate (68%) and data gathering (face-to-face home interviews) make it a very reliable source of information. In addition, special efforts were made to avoid sampling biases (for further details, please see Methods). Moreover, the EAS includes information on CP and disability, which is not available in other important population health surveys, such as the Spanish National Health Survey (www.msssi.gob.es) or the European Health Interview Survey (http://ec.europa.eu/eurostat). It also gathers a large amount of information besides information on CP. For example, information on other diseases, activity limitations, general and employment health and on usage of healthcare services that permit a comprehensive analysis of CP and associated factors. Andalusia, our sampling region, is the most populated (8 399 618 people) and the second largest in area of the 19 regions in Spain. It is also the fifth most populated region in Europe, and it is as populated as other European countries such as Austria or Switzerland. Moreover, we extrapolated the estimations of the DCP prevalence from Andalusia to Spain by applying calibration adjustments. They provide a more accurate estimation and a more valid one when there is non-coverage bias.22 Thus, calibration adjustments increased the validity of generalisation of DCP prevalence from a smaller area, that is, Andalusian region, to a larger one, that is, Spain. Due to the fact that Spain and Andalusia have different sociocultural and economic characteristics, we considered sex and age as calibration variables and educational level and employment status. All those auxiliary variables are considered in the new calibrated weights. They include information from the Andalusian sample as well as from the Spanish census.28 Thus, the extrapolated prevalence of DCP from Andalusia to Spain is representative, at least, for all those variables.22 In addition, the fact that those variables were associated with the study variable (DCP) provided better results in terms of accuracy and validity of the estimations (that is shown in the multivariate model). This statistical method ensures that survey estimates are coherent with those already in the public domain, while simultaneously reducing sampling error and non-coverage or non-response bias.22 51 When compared with the most important surveys published on CP,1 3 5 47 our study is of the same quality and scope but of a higher level than other surveys on DCP.
Conclusions
In summary, the characteristics of chronic pain have been widely studied but without considering whether it disables or not. Our study demonstrates that a population with disabling chronic pain is the one that shows really statistically significant differences. Indeed, very few statistically significant differences were found between the nCP and nDCP populations. DCP is an important public health problem49 that affects a large proportion of the general adult population (11.36% according to our study) with elevated multimorbidity. It has a strong association with social determinants of health (eg, disfavoured or vulnerable social status, impaired quality of health or poor health habits). Moreover, it is a highly relevant issue for health systems49 (DCP almost doubles the health services usage compared with nDCP, especially in primary care).52 Its consequences directly affect partners, families and friends. Therefore, it is a disease that could affect medical practical or political health initiatives, as well as future research areas. Also, the association between DCP and mental disorders highlights the need for psychosocial services in the management of chronic pain. Finally, our study contributes to knowledge on this issue and provides evidence of the need to advance in the application of simple tools for the identification of individuals with DCP. Future research efforts, healthcare and social interventions should focus on this population and on the prevention of future disability in individuals with nDCP.
bmjopen-2017-020913supp001.pdf (130.2KB, pdf)
bmjopen-2017-020913supp002.pdf (119.8KB, pdf)
bmjopen-2017-020913supp004.pdf (76.4KB, pdf)
Supplementary Material
Footnotes
Contributors: AC-L conceived the original idea with the participation of JAGdH and MAC-B. AC-L designed the analysis plan alongside LG-F. Statistical analysis was conducted by LG-F and subsequently by AC-L. AC-L developed the first version of the manuscript in collaboration with MAC-B for the introduction and discussion and with LG-F for the methodology. All authors participated in the writing of subsequent versions and approved the final article.
Funding: The Andalusian Health Survey, the source of information in this study, is funded by the Ministry of Health of the Junta de Andalucía, Spain.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The EAS was supervised and approved by the review board of the General Secretariat of Quality and Public Health in the Health Ministry of the Andalusian Regional Government.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Other tables, analyses, statistics and R code not included in the present article are available on demand.
References
- 1. Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med 2009;10:918–29. 10.1111/j.1526-4637.2009.00655.x [DOI] [PubMed] [Google Scholar]
- 2. Johannes CB, Le TK, Zhou X, et al. . The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain 2010;11:1230–9. 10.1016/j.jpain.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 3. Langley PC, Ruiz-Iban MA, Molina JT, et al. . The prevalence, correlates and treatment of pain in Spain. J Med Econ 2011;14:367–80. 10.3111/13696998.2011.583303 [DOI] [PubMed] [Google Scholar]
- 4. Kennedy J, Roll JM, Schraudner T, et al. . Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. J Pain 2014;15:979–84. 10.1016/j.jpain.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 5. Breivik H, Collett B, Ventafridda V, et al. . Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 6. Blyth FM, March LM, Brnabic AJ, et al. . Chronic pain in Australia: a prevalence study. Pain 2001;89:127–34. 10.1016/S0304-3959(00)00355-9 [DOI] [PubMed] [Google Scholar]
- 7. Sakakibara T, Wang Z, Paholpak P, et al. . A comparison of chronic pain prevalence in Japan, Thailand, and myanmar. Pain Physician 2013;16:603–8. [PubMed] [Google Scholar]
- 8. Wong WS, Fielding R. Prevalence and characteristics of chronic pain in the general population of Hong Kong. J Pain 2011;12:236–45. 10.1016/j.jpain.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 9. Maia Costa Cabral D, Sawaya Botelho Bracher E, Dylese Prescatan Depintor J, et al. . Chronic pain prevalence and associated factors in a segment of the population of São Paulo City. J Pain 2014;15:1081–91. 10.1016/j.jpain.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 10. Dueñas M, Salazar A, Ojeda B, et al. . A nationwide study of chronic pain prevalence in the general spanish population: identifying clinical subgroups through cluster analysis. Pain Med 2015;16:811–22. 10.1111/pme.12640 [DOI] [PubMed] [Google Scholar]
- 11. Eriksen J, Jensen MK, Sjøgren P, et al. . Epidemiology of chronic non-malignant pain in Denmark. Pain 2003;106:221–8. 10.1016/S0304-3959(03)00225-2 [DOI] [PubMed] [Google Scholar]
- 12. Dominick CH, Blyth FM, Nicholas MK. Unpacking the burden: understanding the relationships between chronic pain and comorbidity in the general population. Pain 2012;153:293–304. 10.1016/j.pain.2011.09.018 [DOI] [PubMed] [Google Scholar]
- 13. McEwen BS, Stress MBS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 14. Raftery MN, Sarma K, Murphy AW, et al. . Chronic pain in the Republic of Ireland--community prevalence, psychosocial profile and predictors of pain-related disability: results from the Prevalence, Impact and Cost of Chronic Pain (PRIME) study, part 1. Pain 2011;152:1096–103. 10.1016/j.pain.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 15. Azevedo LF, Costa-Pereira A, Mendonça L, et al. . Epidemiology of chronic pain: a population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J Pain 2012;13:773–83. 10.1016/j.jpain.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 16. Reid MC, Eccleston C, Pillemer K. Management of chronic pain in older adults. BMJ 2015;350:h532 10.1136/bmj.h532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO. International classification of functioning, disability, and health (ICF). World Health Organization. Switzerland: Geneva, 2007. http://www.who.int/classifications/icf/en/ (Accessed 17 Oct 2017). [Google Scholar]
- 18. Blyth FM, March LM, Brnabic AJ, et al. . Chronic pain and frequent use of health care. Pain 2004;111:51–8. 10.1016/j.pain.2004.05.020 [DOI] [PubMed] [Google Scholar]
- 19. Sánchez-Cruz JJ. GarcíaFernándezLl, Mayoral Cortés JM. Andalusian Health Survey, 2011-12. Consejería de Igualdad, Salud y Políticas Sociales 2013. http://goo.gl/2BYFhe (Accessed 3 Aug 2017). [Google Scholar]
- 20. Álvarez-González J, Ayuso-Fernández A, Caba-Barrientos F. Plan Andaluz de atención a las personas con dolor: 2010–2013. Consejería de Salud: Sevilla, 2010. http://www.juntadeandalucia.es/export/drupaljda/plan_atencion_dolor-1_0.pdf (Accessed 3 Aug 2017). [Google Scholar]
- 21. International Association for the Study of Pain (IASP). Washington. IASP Taxonomy: Pain Terms 2004. http://www.iasp-pain.org/Taxonomy?navItemNumber=576#Pain (Accessed 3 Aug 2017). [Google Scholar]
- 22. Cabrera-León A, Lopez-Villaverde V, Rueda M, et al. . Calibrated prevalence of infertility in 30- to 49-year-old women according to different approaches: a cross-sectional population-based study. Hum Reprod 2015;30:2677–85. 10.1093/humrep/dev226 [DOI] [PubMed] [Google Scholar]
- 23. Tillé Y, Matei A; R Package sampling. survey sampling. 2015;76 https://cran.r-project.org/web/packages/sampling/sampling.pdf. [Google Scholar]
- 24. Escobar-Lopez E, Barrios-Zamudio E; SamplingVarEst. Sampling Variance Estimation. 2015. https://cran.r-project.org/web/packages/samplingVarEst/samplingVarEst.pdf (Accessed 5 May 2018).
- 25. Miettinen OS, Cook EF. Confounding: essence and detection1. Am J Epidemiol 1981;114:593–603. 10.1093/oxfordjournals.aje.a113225 [DOI] [PubMed] [Google Scholar]
- 26. Menard S. Coefficients of determination for multiple logistic regression analysis. Am Stat 2000;54:17–24. [Google Scholar]
- 27. Lumley T. Complex Surveys: A Guide to Analysis Using R. Hoboken: Wiley, 2010. [Google Scholar]
- 28. Population and Housing Census. National Statistics Institute (INE, Spanish acronym). 2011. http://www.ine.es/en/censos2011_datos/cen11_datos_inicio_en.htm (Accessed 3 Aug 2017).
- 29. Häuser W, Schmutzer G, Hilbert A, et al. . Prevalence of Chronic Disabling Noncancer Pain and Associated Demographic and Medical Variables: A Cross-Sectional Survey in the General German Population. Clin J Pain 2015;31:886–92. 10.1097/AJP.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 30. Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ 2014;348:f7656 10.1136/bmj.f7656 [DOI] [PubMed] [Google Scholar]
- 31. Reitsma ML, Tranmer JE, Buchanan DM, et al. . The prevalence of chronic pain and pain-related interference in the Canadian population from 1994 to 2008. Chronic Dis Inj Can 2011;31:157–64. [PubMed] [Google Scholar]
- 32. Bingefors K, Isacson D, Epidemiology ID. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain--a gender perspective. Eur J Pain 2004;8:435–50. 10.1016/j.ejpain.2004.01.005 [DOI] [PubMed] [Google Scholar]
- 33. Scherer M, Hansen H, Gensichen J, et al. . Association between multimorbidity patterns and chronic pain in elderly primary care patients: a cross-sectional observational study. BMC Fam Pract 2016;17:68 10.1186/s12875-016-0468-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114–29. 10.1016/j.neuroscience.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organization. Geneva 1994. http://apps.who.int/iris/bitstream/10665/39142/1/WHO_TRS_843_eng.pdf (Accessed 17 Oct 2017). [PubMed] [Google Scholar]
- 36. Kraus VB, Blanco FJ, Englund M, et al. . Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage 2015;23:1233–41. 10.1016/j.joca.2015.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown AK, Conaghan PG, Karim Z, et al. . An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 2008;58:2958–67. 10.1002/art.23945 [DOI] [PubMed] [Google Scholar]
- 38. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol 2000;27:1513. [PubMed] [Google Scholar]
- 39. Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (MD): Office of the Surgeon General (US); 2004. 5, The Burden of Bone Disease. https://www.ncbi.nlm.nih.gov/books/NBK45502/. [PubMed]
- 40. Zota AR, Shenassa ED M-F. Allostatic load amplifies the effect of blood lead levels on elevated blood pressure among middle-age U. S.: a cross-sectional study. Environ Health Persp 2013;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olsen RB, Bruehl S, Nielsen CS, ChS N, et al. . Hypertension prevalence and diminished blood pressure-related hypoalgesia in individuals reporting chronic pain in a general population: the Tromsø study. Pain 2013;154:257–62. 10.1016/j.pain.2012.10.020 [DOI] [PubMed] [Google Scholar]
- 42. Ohayon MM, Stingl JC. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res 2012;46:444–50. 10.1016/j.jpsychires.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 43. Gerrits MM, Vogelzangs N, van Oppen P, et al. . Impact of pain on the course of depressive and anxiety disorders. Pain 2012;153:429–36. 10.1016/j.pain.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 44. Loerbroks A, Bosch JA, Mommersteeg PM, et al. . The association of depression and angina pectoris across 47 countries: findings from the 2002 World Health Survey. Eur J Epidemiol 2014;29:507–15. 10.1007/s10654-014-9926-z [DOI] [PubMed] [Google Scholar]
- 45. Chopra K, Arora V. An intricate relationship between pain and depression: clinical correlates, coactivation factors and therapeutic targets. Expert Opin Ther Targets 2014;18:159–76. 10.1517/14728222.2014.855720 [DOI] [PubMed] [Google Scholar]
- 46. Leonardi M, Bickenbach J, Ustun TB, et al. . The definition of disability: what is in a name? Lancet 2006;368:1219–21. 10.1016/S0140-6736(06)69498-1 [DOI] [PubMed] [Google Scholar]
- 47. Gibson SJ, Lussier D. Prevalence and relevance of pain in older persons. Pain Med 2012;13 Suppl 2:S23–S26. 10.1111/j.1526-4637.2012.01349.x [DOI] [PubMed] [Google Scholar]
- 48. Smith BH, Elliott AM, Chambers WA, et al. . The impact of chronic pain in the community. Fam Pract 2001;18:292–9. 10.1093/fampra/18.3.292 [DOI] [PubMed] [Google Scholar]
- 49. Blyth FM, Van Der Windt DA, Croft PR. Chronic Disabling Pain: A Significant Public Health Problem. Am J Prev Med 2015;49:98–101. 10.1016/j.amepre.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 50. Ph S. Cross sectional studies: advantages and disadvantages. BMJ 2014;348:g2276. [Google Scholar]
- 51. Cabrera-León A, Rueda M, Cantero-Braojos M. “Calibrated prevalence of disabling chronic pain: a face-to face cross-sectional population study in Southern Spain”. BMJ Open 2017;7:e014033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cabrera-León A, Cantero-Braojos MA. “Impact of disabling chronic pain: results of a cross-sectional population study with face-to-face interview”. Aten Prim. In Press;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dapcich V, Salvador G, Ribas L, et al. . Sociedad Española de Nutrición Comunitaria. 2004. http://aesan.msssi.gob.es/AESAN/docs/docs/publicaciones_estudios/nutricion/guia_alimentacion.pdf (Accessed 3 Aug 2017).
- 54. Vilagut G, Valderas JM, Ferrer M, et al. . [Interpretation of SF-36 and SF-12 questionnaires in Spain: physical and mental components]. Med Clin 2008;130:726–35. [DOI] [PubMed] [Google Scholar]
- 55. Verbrugge LM. A global disability indicator. J Aging Stud 1997;11:337–62 http://aesan.msssi.gob.es/AESAN/docs/docs/publicaciones_estudios/nutricion/guia_alimentacion.pdf 10.1016/S0890-4065(97)90026-8 [DOI] [Google Scholar]
- 56. Ayala A, Rodríguez-Blázquez C, Frades-Payo B, et al. . [Psychometric properties of the Functional Social Support Questionnaire and the Loneliness Scale in non-institutionalized older adults in Spain]. Gac Sanit 2012;26:317–24. 10.1016/j.gaceta.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 57. Chilet-Rosell E, Álvarez-Dardet C, Domingo-Salvany A. [Use of Spanish proposals for measuring social class in health sciences]. Gac Sanit 2012;26:566–9 http://scielo.isciii.es/pdf/gs/v26n6/original_breve1.pdf 10.1016/j.gaceta.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 58. Ewing JA, alcoholism D. The CAGE questionnaire. JAMA 1984;252:1905–7. [DOI] [PubMed] [Google Scholar]
- 59. WHO. Body Mass Classification. World Health Organization. Switzerland: Geneva, 2015. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (Accessed 3 Aug 2017). [Google Scholar]
- 60. Nübling M, Burr H, Moncada S, et al. . COPSOQ International Network: Co-operation for research and assessment of psychosocial factors at work. Public Health Forum 2014;22:e1–18. 10.1016/j.phf.2013.12.019 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-020913supp003.pdf (77.2KB, pdf)
bmjopen-2017-020913supp001.pdf (130.2KB, pdf)
bmjopen-2017-020913supp002.pdf (119.8KB, pdf)
bmjopen-2017-020913supp004.pdf (76.4KB, pdf)