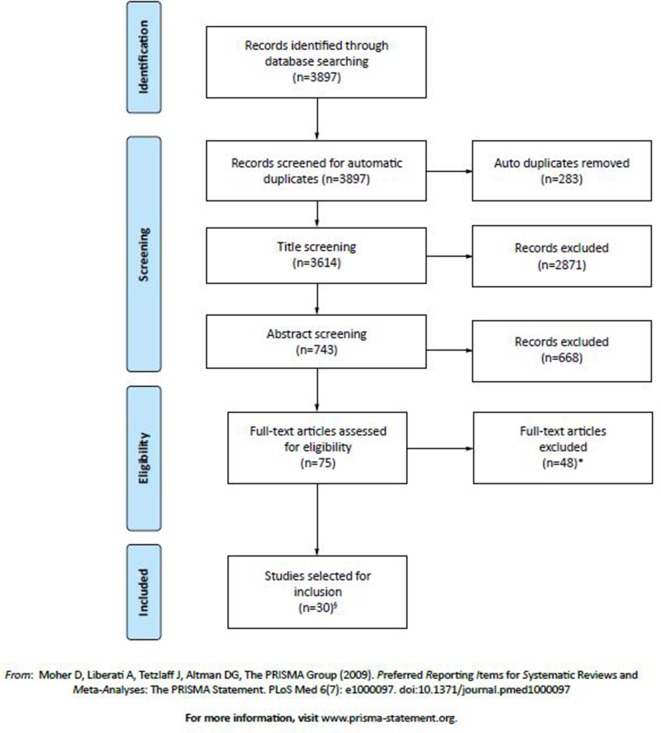
Figure 1.
Search strategy and selection of studies presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. *Studies were excluded for the following reasons: outcome measure(s) of persistence and/or adherence, relevant to this systematic review (such as medication possession rate, proportion of days covered, discontinuation rate), were not presented within the full text of the article (n=17); adherence/persistence data were drawn from surveys, interviews or self-reports (n=13); cohort contained a portion of patients under 18 years of age (who could not be removed or isolated from results/data) (n=7); participants had prior awareness/knowledge of partaking in a study related to overactive bladder (OAB) medication (ie, open-label extension to a study or prior written consent) (n=6); a full article text was not available (ie, only a conference abstract) or the full text was not in English (n=4); or non-oral OAB medications were included within the presented results (and could not be removed or isolated from results/data) (n=1). §Three of these studies were identified by reviewing reference lists of included studies and relevant systematic literature reviews.