Abstract
2-Hydroxy-oleic acid (2OHOA) is a potent anticancer drug that induces cancer cell cycle arrest and apoptosis. Previous studies have suggested that 2OHOA's anticancer effect is mediated by SMS activation in cancer cells, including A549 and U118 cells. To confirm this phenomenon, in this study, we treated both A549 and U118 cells with 2OHOA and measured SMS activity. To our surprise, we found neither 2OHOA-mediated SMS activation nor sphingomyelin accumulation in the cells. However, we noted that 2OHOA significantly reduces phosphatidylcholine in these cells. We also did not observe 2OHOA-mediated SMS activation in mouse tissue homogenates. Importantly, 2OHOA inhibited rather than activated recombinant SMS1 (rSMS1) and rSMS2 in a dose-dependent fashion. Intra-gastric treatment of C57BL/6J mice with 2OHOA for 10 days had no effects on liver and small intestine SMS activities and plasma sphingomyelin levels. The treatment inhibited lysophosphatidylcholine acyltransferase (LPCAT) activity, consistent with the aforementioned reduction in plasma phosphatidylcholine. Because total cellular phosphatidylcholine is used as a predictive biomarker for monitoring tumor responses, the previously reported 2OHOA-mediated cancer suppression could be related to this phosphatidylcholine reduction, which may influence cell membrane structure and properties. We conclude that 2OHOA is not a SMS activator and that its anticancer property may be related to an effect on phosphatidylcholine metabolism.
Keywords: sphingolipid, phospholipid, lipid, lipid metabolism, cancer, 2-hydroxy-oleic acid (2OHOA), anti-cancer drug, lysophosphatidylcholine acyltransferase (LPCAT), sphingomyelin synthase (SMS), sphingomyelin, phosphatidylcholine
Introduction
2-Hydroxy-oleic acid (2OHOA)3 is a potent anticancer drug that induces cancer cell cycle arrest (1), differentiation (2), and death (3–5). The European Medicines Agency has designated 2OHOA as an orphan drug for the treatment of glioma.
However, the underlying mechanisms leading to cancer cell death remain largely unknown, although it has been reported that the effect was mediated by activation of sphingomyelin synthase (SMS) in cancer cells and accumulation of cell sphingomyelin levels (6).
The biochemical synthesis of SM occurs through the action of serine palmitoyltransferase, 3-ketosphinganine reductase, ceramide synthase, dihydroceramide desaturase, and SMS (7). SMS catalyzes the conversion of ceramide to sphingomyelin. The SMS gene family consists of three members, SMS1, SMS2, and SMS-related protein (SMSr). SMS1 is found in the trans-Golgi complex, whereas SMS2 is predominantly found in the plasma membranes (8, 9). SMSr, the third member of the gene family, has no SMS activity but catalyzes the synthesis of ceramide-phosphoethanolamine in the ER lumen (8, 10). We and another research group reported that systemic SMS1 KO exhibited moderate neonatal lethality (11, 12) (i.e. 25% of homozygotes die during the first 3 weeks; the remainder can grow to adulthood). On the other hand, SMS2 KO mice display no obvious abnormalities and grow to adulthood (13). We and others have suggested that SMS2 might be a therapeutic target for metabolic diseases, including type 2 diabetes, fatty liver, and atherosclerosis (14–20). However, the potential effect of 2OHOA on SMS activation (6) could compromise the effort of SMS2 inhibitor exploration (21). In this study, we re-evaluated the effect of 2OHOA on SMS activity, using the same cells reported by previous studies (6), and we could not repeat what had been reported. We further explored a potential 2OHOA-mediated anti-cancer mechanism.
Results
2OHOA does not activate SMS activity
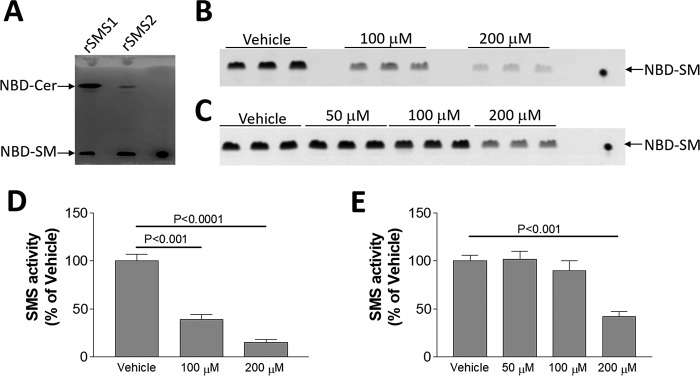
We re-evaluated the effect of 2OHOA on SMS. To our surprise, we did not find the same effect of 2OHOA reported before (6). We first incubated 2OHOA (200 μm) with mouse liver homogenate and then measured SMS activity: generation of NBD-SM from NBD-ceramide. We not only did not observe the activation, but also observed some inhibition, although it did not reach statistical significance (Fig. 1, A and B). Moreover, we performed the same analysis on homogenates from either HEK293 cells or HeLa cells, and we observed a significant reduction instead of induction on SMS activity in a dose-dependent fashion (Fig. 1, C and D).
Figure 1.
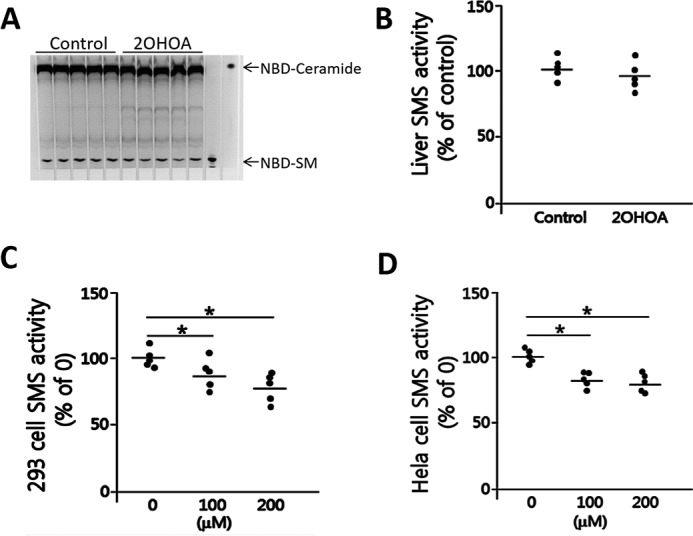
2OHOA does not activate SMS (tissue homogenates). A, liver SMS activity measurement. Mouse liver homogenate was incubated with 2OHOA (200 μm) together with NBD-ceramide. NBD-SM, the product of SMS, was separated by TLC. B, quantification of SMS activity. C, HEK293 cell SMS activity measurement with the indicated dose of 2OHOA. D, HeLa cell SMS activity measurement with the indicated dose of 2OHOA. Values are mean ± S.D. (error bars), n = 5; *, p < 0.05.
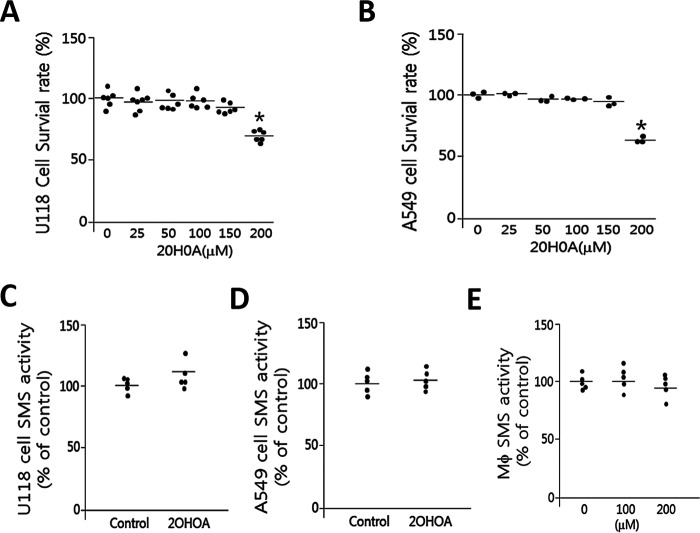
It has been reported that the treatment of 2OHOA (200 μm) on U118 and A549, two cancer cell lines, could stimulate SMS activity in both cells, leading to cell death (6). We next re-evaluated this assay. We first determined the cell survival rate after 2OHOA treatment in different doses. Indeed, 200 μm 2OHOA could significantly reduce the survival rate of U118 cells (Fig. 2A) and A549 cells (Fig. 2B). We treated U118 and A549 cells with 2OHOA (200 μm) and then used the cell homogenates for SMS activity analysis. Again, we did not find significant changes of SMS activity in either cell line (Fig. 2, C and D). Further, we treated mouse primary macrophages with 200 μm 2OHOA, and we again did not find any significant changes in SMS activity (Fig. 2E). These results indicate that 2OHOA cannot stimulate SMS activity in our tested cancer cells and macrophages.
Figure 2.
2OHOA does not activate SMS (ex vivo). U118 cells (A) and A549 cells (B) were treated with different concentration of 2OHOA for 24 h, and cell mortality was monitored by an MTT assay. Cells were treated with 200 μm 2OHOA, and then NBD-ceramide was added to the cell culture medium and incubated for 30 min. SMS activity was measured in U118 cells (C), A549 cells (D), and macrophage cells (E). Values are mean ± S.D. (error bars), n = 3–5.
To directly test the effect of 2OHOA, we transfected insect cells with SMS1-Strep tag and SMS2-Strep tag and purified both proteins, which have high SMS activity (Fig. 3A). We then incubated the purified proteins with different concentration of 2OHOA for 2 h, and we found that 2OHOA has a dose-dependent inhibition of SMS1 (Fig. 3, B and D) and SMS2 (Fig. 3, C and E) instead of activation.
Figure 3.
2OHOA does not activate purified SMS. SMS1-His tag and SMS2-His tag were purified from transfected insect cells (see “Experimental procedures”). A, SMS activity of SMS1-His tag and SMS2-His tag. SMS1-His tag and SMS2-His tag (1 μg) were incubated with 200 μm 2OHOA for 2 h, and then SMS activity was measured by adding NBD-ceramide. NBD-SM was the product. B, effect of 2OHOA on SMS1-His tag. C, effect of 2OHOA on SMS2-His tag. D and E, quantification of SMS activity by SMS1-His tag and SMS2-His tag. Values are mean ± S.D. (error bars), n = 3.
2OHOA inhibits lysophosphatidylcholine acyltransferase (LPCAT) activity
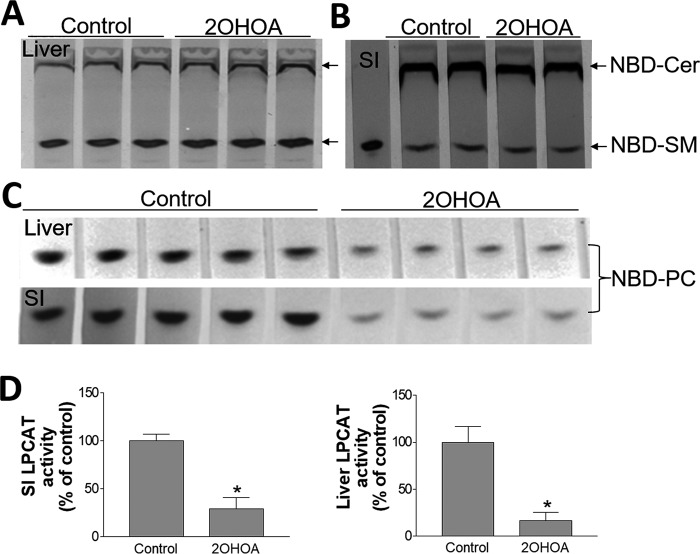
2OHOA is an analog of oleic acid that can be used as a substrate for LPCAT activity. It seems to be reasonable that 2OHOA could interfere with LPCAT activity by competing with acyl-CoA. Indeed, when we utilized tissue homogenates from mouse liver, lung, brain, and testis to test LPCAT activity, using oleoyl-CoA, we found that 2OHOA inhibits LPCAT activity in a dose-dependent manner (Fig. 4, A–D). We then treated both mouse liver and small intestine homogenates with 200 μm 2OHOA and oleoyl-CoA, and we found a dramatic reduction of LPCAT activity (Fig. 4E). However, when we used arachidonyl-CoA as a substrate, the liver inhibition did not reach to statistical significance (Fig. 4F), indicating that 2OHOA may have minimal effect on LPCAT3, one of the four isoforms of LPCAT. Arachidonyl-CoA is the optimal substrate for LPCAT3 (22). Based on the structure of 2OHOA, most likely, it could compete with the substrate of LPCAT (i.e. oleoyl-CoA and some other acyl-CoA). Potentially, any biochemistry reaction with oleoyl-CoA as a substrate could be influenced by 2OHOA treatment. Thus, it is not likely that 2OHOA could influence mRNA and protein levels of SMS1, SMS2, and LPCAT.
Figure 4.
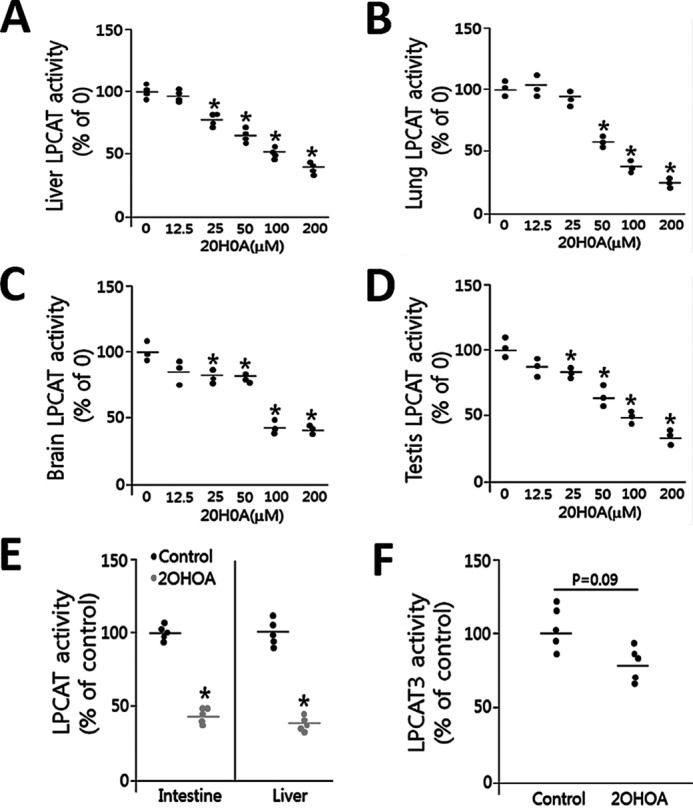
2OHOA inhibits LPCAT activity (tissue homogenates). Difference concentrations of 2OHOA were added to tissue homogenates. Tissue total LPCAT activity (using oleoyl-CoA and NBD-lyso-PC) was measured. Liver (A), lung (B), brain (C), testis (D), and small intestine and liver homogenates (E) were treated with 200 μm 2OHOA, and LPCAT activity (using oleoyl-CoA and NBD-lyso-PC) was measured. F, liver homogenates were treated with 200 μm 2OHOA, and LPCAT3 activity (using arachidonyl-CoA and NBD-lyso-PC) was measured. Values are mean ± S.D. (error bars), n = 3–5. *, p < 0.05.
2OHOA treatment has no effect on cellular and membrane SM levels but significantly reduces phosphatidylcholine (PC) levels
It has been reported that the treatment of 2OHOA (200 μm) on U118 and A549 cells caused SM accumulation owing to SMS activation (6). To re-evaluate this, we extracted the lipids from A549 cells after 2OHOA (200 μm) treatment and measured PC and SM subspecies using LC/MS/MS. We found that almost all tested PCs were significantly reduced (Table 1). However, no tested SMs had any significant changes (Table 2), except 18:0, which is a minor SM (less than 3% of all tested SM). These results again indicated that 2OHOA is not a SMS activator.
Table 1.
Changes in A549 cell PC species after 2OHOA treatment
After treatment with 2OHOA (200 μm), cells were homogenized, and protein concentrations were measured. Cell homogenates with the same protein concentration were used for lipid extraction. PC peak areas were obtained after LC/MS/MS analysis. Values (arbitrary units) are mean ± S.D., n = 3. NS, not significant.
| PC | Control | 2OHOA | p value |
|---|---|---|---|
| 16:0/16:0 | 15.31 ± 1.18 | 15.87 ± 0.98 | NS |
| 16:0/18:1 | 66.29 ± 4.58 | 53.84 ± 4.07 | <0.02 |
| 18:0/20:4 | 1.41 ± 0.02 | 0.89 ± 0.05 | <0.001 |
| 18:1/16:0 | 47.14 ± 4.14 | 39.07 ± 3.09 | <0.05 |
| 18:1/18:0 | 4.93 ± 0.38 | 4.20 ± 0.22 | <0.05 |
Table 2.
Changes in A549 cell SM species after 2OHOA treatment
After treatment of 2OHOA (200 μm), cells were homogenized, and protein concentrations were measured. Cell homogenates with the same protein concentration were used for lipid extraction. SM peak areas were obtained after LC/MS/MS analysis. Values (arbitrary units) are mean ± S.D., n = 3. NS, not significant.
| SM | Control | 2OHOA | p value |
|---|---|---|---|
| 16:0 | 2.62 ± 0.26 | 2.75 ± 0.43 | NS |
| 18:0 | 0.17 ± 0.02 | 0.21 ± 0.02 | <0.05 |
| 18:1 | 0.04 ± 0.01 | 0.04 ± 0.00 | NS |
| 24:0 | 0.89 ± 0.10 | 0.95 ± 0.08 | NS |
| 24:1 | 2.67 ± 0.25 | 2.51 ± 0.07 | NS |
To further investigate the effect of 2OHOA on cell plasma membrane, we studied lysenin-mediated cell lysis. Lysenin is a SM-specific cytotoxin. Lysenin recognizes plasma membrane SM only when it forms aggregates or microdomains and then causes cell lysis (23). If 2OHOA could indeed induce SM accumulation on the plasma membrane, as suggested previously (6), then the lysenin should cause more cell death. However, we did not observe any changes in lysenin-mediated A549 cell mortality with or without 2OHOA treatment (Fig. 5), suggesting no SM increasing on the plasma membrane. These results again indicated that 2OHOA is not a SMS activator.
Figure 5.
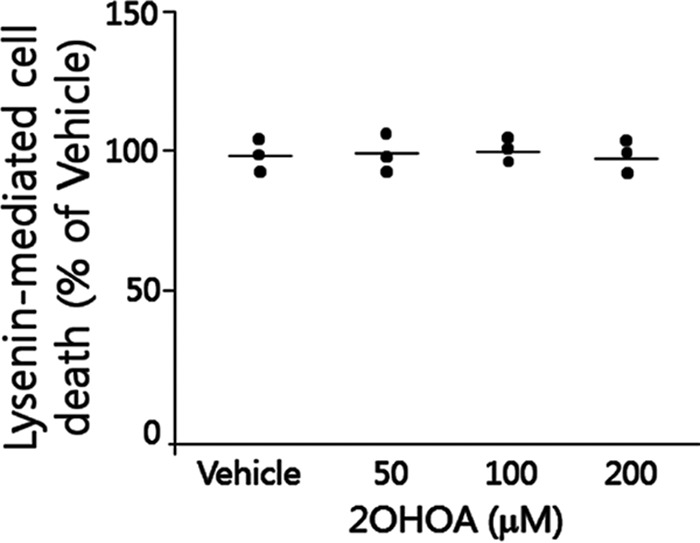
Effect of lysenin on A549 cells treated with 2OHOA. A549 cells were treated with 200 μm 2OHOA or vehicle for 18 h and then treated with lysenin (200 ng/ml) for 2 h. The cell mortality was monitored as described under “Experimental Procedures.” Values are mean ± S.D. (error bars), n = 3.
2OHOA inhibits LPCAT activity: in vivo study
We next investigated in vivo effects of 2OHOA. We treated C57BL/6 mice with 2OHOA (600 mg/kg, intragastrically) once daily for 7 days. We found that the treatment had no effect on liver and small intestine SMS activity (Fig. 6, A and B), but significantly reduced liver and small intestine LPCAT activity, when oleoyl-CoA was used (Fig. 6, C and D). Moreover, 2OHOA significantly decreased plasma PC, cholesterol, and triglyceride levels (Fig. 7, A, C, and D). We also measured plasma subspecies of PC, using LC/MS/MS, and found that almost all PCs were significantly reduced (Table 3). Importantly, plasma SM levels were reduced instead of increased (Fig. 7B), although it did not reach statistical significance. We also measured plasma subspecies of SM and found that SM16:0 was significantly reduced, whereas two other major species, SM 24:0 and SM24:1, were reduced, but did not reach to statistical significance (Table 4). We also observed that two minor SMs (SM18:0 and SM18:1) were significantly increased, but they only compose about 3% of total plasma SM levels. These results indicate that 2OHOA has a significant effect on plasma PC but not SM, suggesting, again, that 2OHOA is not a SMS activator.
Figure 6.
The in vivo effect of 2OHOA. A and B, effect on liver and small intestine (SI) total SMS activity (using NBD-ceramide (Cer) as substrate). C and D, effect on liver and SI total LPCAT activity (using oleoyl-CoA and NBD-lyso-PC as substrates) and quantification. Values are mean ± S.D. (error bars), n = 4–5. *, p < 0.001.
Figure 7.
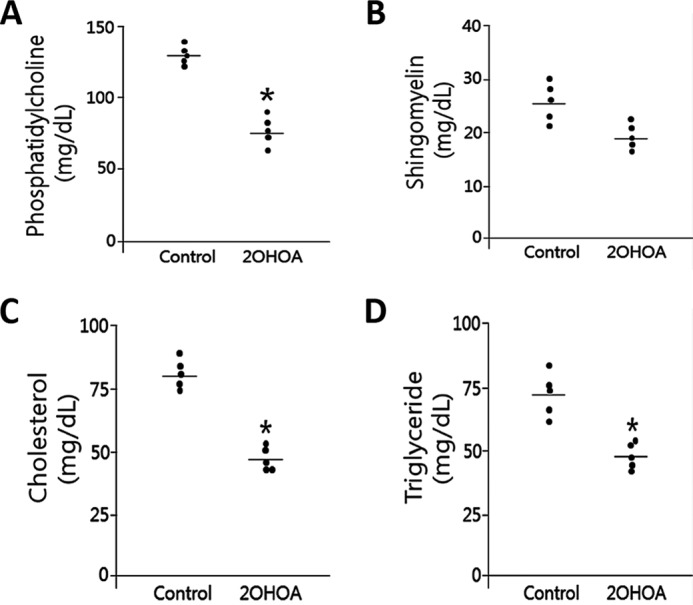
The effect of 2OHOA on plasma lipid levels. All plasma lipids were measured by colorimetric methods. A, phosphatidylcholine; B, sphingomyelin; C, total cholesterol; D, triglyceride. Values are mean ± S.D. (error bars), n = 5. *, p < 0.01.
Table 3.
Changes in plasma PC species after 2OHOA treatment
Values are mean ± S.D. n = 4. NS, not significant.
| PC | Control | 2OHOA | p value |
|---|---|---|---|
| μg/ml | μg/ml | ||
| 34:1 | 262 ± 93 | 134 ± 13 | <0.01 |
| 16:0/18:2 | 895 ± 205 | 387 ± 73 | <0.01 |
| 36:1 | 92 ± 24 | 49 ± 18 | <0.01 |
| 18:1/18:1 | 549 ± 78 | 265 ± 105 | <0.01 |
| 36:3 | 225 ± 28 | 86 ± 5 | <0.001 |
| 36:4 | 260 ± 63 | 87 ± 9 | <0.01 |
| 18:0/20:4 | 123 ± 34 | 51 ± 8 | <0.01 |
| 38:4 | 123 ± 34 | 51 ± 8 | <0.01 |
| 38:5 | 95 ± 28 | 38 ± 6 | <0.01 |
| 16:0/22:6 | 292 ± 89 | 174 ± 28 | <0.05 |
| 40:3 | 5 ± 2 | 2 ± 1 | NS |
| 40:6 | 81 ± 33 | 57 ± 3 | NS |
Table 4.
Changes in plasma SM species after 2OHOA treatment
Values are mean ± S.D. n = 4. NS, not significant.
| SMs | Control | 2OHOA | p value |
|---|---|---|---|
| μg/ml | μg/ml | ||
| 16:0 | 8.1 ± 0.8 | 6.6 ± 0.2 | <0.05 |
| 16:1 | 1.1 ± 0.3 | 0.8 ± 0.1 | <0.05 |
| 18:0 | 0.6 ± 0.1 | 1.2 ± 0.3 | <0.05 |
| 18:1 | 0.5 ± 0.1 | 0.9 ± 0.3 | <0.05 |
| 18:2 | 0.1 ± 0.0 | 0.2 ± 0.1 | <0.05 |
| 20:0 | 0.3 ± 0.1 | 0.5 ± 0.2 | NS |
| 22:0 | 2.6 ± 1.5 | 2.0 ± 0.6 | NS |
| 24:0 | 3.8 ± 1.3 | 2.7 ± 0.4 | NS |
| 24:1 | 17.1 ± 3.7 | 15.0 ± 3.2 | NS |
Discussion
We have reported that inhibition of SMS activity can reduce atherogenic lipoprotein production and attenuate endotoxin-mediated macrophage inflammation (12, 24, 25). Likewise, SMS inhibition could be a new therapeutic target for the treatment of atherosclerosis. However, based on existing reports, inhibition of SMS seems to result in tumorigenesis, because 2OHOA, a potent anticancer drug, could activate SMS in cancer cells (6). We thus re-evaluated the effect of 2OHOA on SMS.
In this study, we found that 2OHOA treatment 1) has no effect on SMS activation in tested cancer cells, normal cells, and mouse tissue homogenates and has no effect of SM accumulation in cancer cells; 2) inhibits instead of activates rSMS1 and rSMS2 activity in a dose-dependent manner; and 3) can inhibit LPCAT but not LPCAT3 activity and significantly reduces phosphatidylcholine levels.
Although we cannot explain the discrepancy between our results and a previous report, in fact, we repeated the experiments that were reported (6). We treated U118 and A459 cancer cell lines with 2OHOA, and we found significant changes in neither SMS activity (Fig. 2, B and C) nor cellular SM levels (Table 2) and plasma membrane SM levels (Fig. 5), indicating that the anti-cancer property of 2OHOA should not be related with cancer cell SMS activation. However, both our study and the previous study show that 2OHOA treatment can significantly reduce cellular PC levels (Table 1) (6).
Tumor xenografts are a popular model for the study of the action of new anti-tumor drugs. We also noticed that a previous report, which analyzed changes in the lipidome of xenografts after treatment with 2OHOA, showed the induction of two SMs (34:1 and 42:2). Neither the rest of the major SMs nor SMS activity levels were indicated in the tumor xenografts (26). Interestingly, 2OHOA treatment significantly reduced almost all major PCs (16;0/18:1, 16:0/16:0, 16:0/16:1, 16:0/18:2, 18:0/18:2, and 16:0/20:4), although the treatment had an opposite effect on some minor PCs (less than 5% of total PCs) (26). We did not repeat the xenografts; however, we treated C57BL/6 mice with same dose of 2OHOA (600 mg·kg−1) for 10 days, and we found a significant reduction of plasma PCs (Fig. 7A) but not SM (Fig. 7B), which reflects a steady state of PC and SM metabolism (biosynthesis and catabolism).
PCs are first synthesized from glycerol 3-phosphate in the de novo biosynthetic pathway, originally described by Kennedy and Weiss in 1956 (Kennedy pathway) (27), and undergo maturation in the remodeling pathway, as reported by Lands in 1958 (Lands' cycle) (28). The PC remodeling consists of two steps: the deacylation step, which is catalyzed by calcium-independent phospholipase A2 (29), and the reacylation step, which is catalyzed by LPCAT (22, 30–33). Inhibition of LPCAT could cause the reduction of phosphatidylcholines, which can be used as a predictive biomarker for monitoring tumor response (21). We further found that the 2OHOA-mediated reduction of PC could be related with inhibition of LPCAT activity with oleoyl-CoA but not arachidonyl-CoA as a substrate, in vitro (Fig. 4, E and F) and in vivo (Fig. 6, C and D). Thus, 2OHOA can inhibit activity of LPCAT but not LPCAT3, which specifically uses arachidonyl-CoA as a substrate (34). Potentially, any biochemistry reaction with oleoyl-CoA as a substrate could be influenced by 2OHOA treatment.
Evidence has accrued that PC, the major phospholipid component of eukaryotic membranes, contributes to both proliferative growth and programmed cell death. On the one hand, the PC content was shown to increase with cell transformation and tumor progression (35–38). However, on the other hand, PC synthesis and degradation plays an important role in cancer apoptosis, reinforcing the central role of PC and its metabolites in determining cell fate (39). Moreover, total cellular phospholipid metabolism can be used as a predictive biomarker for monitoring tumor response (40). Furthermore, 2OHOA-mediated PC changes might influence whole-body metabolism, because metabolic disturbance by altered PC metabolism has been suggested (41).
The 2OHOA-mediated PC reduction and its impact on cancer growth and apoptosis deserves further investigation
The anticancer property of 2OHOA could be related to its influence on cell membrane. Martin et al. (42) reported that 2OHOA increased the packing of ordered domains and decreased the global order of the membrane and subsequently modulated membrane protein–associated signaling. Ibarguren et al. (43) reported that 2OHOA could be related to its modulation on the membrane lipid structure and the proportion of Lo/Ld microdomains. Although both natural fatty acids and 2OHOA exert similar structural effects in the cell membrane lipid bilayer, the low rate of metabolism of 2OHOA may favor its therapeutic effect compared with natural fatty acids. These regulatory effects can cause relevant modifications in the localization of signaling proteins and may be part of the mechanism of action involved in the therapeutic effects exerted by 2OHOA (43).
In conclusion, 2OHOA is not an activator of SMS activity. Its treatment does not increase tissue SM levels but reduces PC levels. The 2OHOA-mediated anti-cancer property deserves further investigation. We believe that SMS2 inhibition is a potential metabolic disease therapeutic approach, which should not be related with cancer generation.
Experimental procedures
Reagents
Dulbecco's modified Eagle's medium was from MULTICELL. Fetal bovine serum and penicillin/streptomycin solution were from Thermo Scientific Fisher Scientific HyClone. 2OHOA was from Toronto Research Chemicals and Avanti Polar Lipids, respectively. We found that both 2OHOAs were identical (data not shown). We used 2OHOA from Toronto Research Chemicals to do all of the experiments and used 2OHOA from Avanti Polar Lipids to confirm some of the experiments. C6-NBD-ceramide and phosphatidylcholine were purchased from Sigma. The BCA protein assay kit was from Cwbiotech.
Mice
Mice (10 weeks old) on a C57BL/6 background were purchased from Jackson Laboratory (Bar Harbor, ME). Experimental mice were housed in a temperature- and humidity-controlled room with a 12/12-h light/dark cycle. Mice were fed a chow diet. Experiments involving mice were conducted with the approval of SUNY Downstate Medical Center institutional animal care and use committee. The procedures followed were in accordance with institutional guidelines.
Cell culture
Human glioma (U118), human non-small lung adenocarcinoma (A549) cells, HEK293 cells, and HeLa cells were obtained from the cell bank, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 100 units/ml penicillin, and 100 mg/ml streptomycin at 37 °C, 5% CO2. Exponential-phase cells were collected for further experiments.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Human non-small lung adenocarcinoma (A549) cells were plated at a density of 1 × 104 cells in 96-well plates, incubated for 24 h, and then exposed to various concentrations of either 2OHOA or staurosporine for another 24 or 48 h. At the end of the treatment, cell viability was determined using the MTT method.
SMS activity measurement
For determination of all SMS activity in cell or tissue homogenate, the cells or tissues were homogenized in 50 mm Tris-HCl, pH 7.5, 1 mm EDTA, 5% sucrose, and 1 mm PMSF and centrifuged at 300 × g for 5 min at 4 °C. Total protein (200 μg) of the homogenate was mixed with C6-NBD-ceramide (4.3 μm) and phosphatidylcholine (95 μm) in the presence or absence of various concentrations of 2OHOA. The mixture was incubated at 37 °C for 2 h, and the reaction was terminated by adding chloroform/methanol (2:1, v/v) and mixing vigorously. The lower organic phase was collected after centrifugation (10,000 × g for 10 min) and dried under nitrogen gas. Lipids were redissolved in 40 μl of chloroform and then were applied to a thin-layer chromatography (TLC) plate. The running solvent was chloroform/MeOH/NH4OH (16:4:1, v/v/v). The fluorescence signal was detected under UV, and the intensity was measured by ImageJ software.
2OHOA treatment and SMS activity assay in cultured cells
A549 or U118 cells were treated with 200 μm 2OHOA (Sigma) for 24 h. The cells were homogenized in assay buffer containing 100 mm Tris-HCl (pH 7.4), 50 mm KCl, 1 mm EDTA, and 1 mm protease inhibitor (PMSF). The homogenate was centrifuged at 2000 rpm for 10 min at 4 °C, and the cell supernatant (200 μg of protein) was mixed in assay buffer containing 100 mm Tris-HCl (pH 7.4), 50 mm KCl, 1 mm EDTA, C6-NBD-ceramide (4.3 μm) (Avanti Polar Lipids), and phosphatidylcholine (95 μm) (Sigma). The mixture was incubated at 37 °C for 2 h, and the reaction was terminated by adding chloroform/methanol (2:1, v/v) and mixing vigorously. The lower organic phase was collected after centrifugation (10,000 × g for 10 min) and dried under nitrogen gas. Lipids were redissolved in 40 μl of chloroform and then were applied to a TLC plate. The running solvent was chloroform/MeOH/NH4OH (16:4:1, v/v/v). The fluorescence signal was detected under UV, and the intensity was measured by ImageJ software.
Alternatively, highly purified SMS1 and highly purified SMS2 were obtained from the Chinese Academy of Sciences (Shanghai, China) and were used for the SMS activity assay. The reaction system contained 100 mm Tris-HCl (pH 7.4), 50 mm KCl, C6-NBD-ceramide (4.3 μm), and phosphatidylcholine (95 μm), 1 mm PMSF, various concentrations of 2OHOA (Sigma), and highly purified SMS1/2. The mixture was incubated at 37 °C for 2 h. Lipids were extracted in chloroform/methanol (2:1, v/v), dried under N2 gas, and separated by TLC using chloroform/MeOH/NH4OH (16:4:1, v/v/v).
LPCAT activity measurement
For determination of all LPCAT activity in tissue homogenates, the tissues were homogenized in 75 mm Tris-HCl, pH 7.5, 15 μm BSA, fatty acid-free (Sigma), and 1 mm protease inhibitor (PMSF). The homogenate was centrifuged at 2000 rpm for 10 min at 4 °C. Total protein (300 μg) of the homogenate was mixed with arachidonoyl-CoA (2.37 mm) (Sigma) and NBD-lysophosphatidylcholine (NBD-lyso-PC; 0.81 mm) (Avanti Polar Lipids) in the presence or absence of various concentrations of 2OHOA. The mixture was incubated at 25 °C for 10 min, and the reaction was terminated by adding chloroform/methanol (1:1, v/v) and mixing vigorously. The lower organic phase was collected after centrifugation (10,000 × g for 10 min) and dried under nitrogen gas. Lipids were redissolved in 40 μl of chloroform and then were applied to a TLC plate. The running solvent was chloroform/MeOH/NH4OH (65:25:4, v/v/v). The fluorescence signal was detected under UV, and the intensity was measured by ImageJ software.
Lysenin-mediated cell mortality measurement
Lysenin-mediated cell mortality was measured as described by us before. Briefly, after treatment with various concentrations of 2OHOA for 24 h, A549 cells were washed with PBS, and 200 ng/ml lysenin in serum-free medium was added in cells and incubated for another 2 h at 37 °C. MTT was used to determine cell viability.
Analysis of lipids by LC-ESI/MS/MS
A549 cells were treated with 200 μm 2OHOA (Sigma) for 24 h. The cells were homogenized in PBS. The homogenate was centrifuged at 1200 rpm for 10 min at 4 °C. Cell pellets were collected to 400 μg of total protein (BCA assay) for lipid extraction. Lipids were extracted in chloroform/methanol (2:1, v/v), dried under N2 gas.
The quantitation of lipids in plasma was accomplished using LC electrospray ionization tandem MS. LC/ESI/MS/MS analysis of phosphatidylcholine and sphingomyelin was performed using an AB Sciex 6500 mass spectrometer with an ESI probe and interfaced with an LC system in the positive multiple-reaction monitoring mode. The UHPLC system consisted of an Agilent 1290 binary pump, thermostat, TCC, and sampler. The injection volume was 2 μl for lipid extracts. Lipid extracts were chromatographically resolved using a Halo Penta Hilic UHPLC column, 2.1 × 150 mm, 2.7 μm (Mac-Mod (Chadds Ford, PA), PN: 92812-705). Mobile phase A was acetonitrile, methanol, 0.5% formic acid, 5 mm ammonium formate (95:5, by volume ratio, v/v). Mobile phase B was water, 0.5% formic acid, 5 mm ammonium formate. The solvent flow rate was 0.7 ml/min. The valve, sample loop, and needle were washed with 50% acetonitrile, 50% methanol for 25 s. The column temperature was kept at 50 °C. Internal standard mixture was added to the samples. The samples were then reconstituted in acetonitrile, methanol, 5 mm ammonium formate (70:30 by volume ratio, v/v). Lipid levels were quantified by the ratio of analyte and internal standard, and calibration curves were obtained by serial dilution of a mixture of lipid standards. Pure synthetic standards of phosphatidylcholine and sphingomyelin were purchased from Avanti Lipids. Deuterated synthetic standards were synthesized internally at Eli Lilly and Company.
The quantitation of lipids in A549 cells was performed using an Ultra-Triple quadrupole mass spectrometer (AB SCIEX Triple TOF 5600) equipped with an ESI probe and interfaced with the Eksigent LC100 system. Lipid extracts were separated with an XBridge Peptide BEH C18 column (Waters). Mobile phase A was 10 mm ammonium acetate, 0.1% formic acid, 99.9% water, and mobile phase B was 10 mm ammonium acetate, 0.1% formic acid, 49.95% acetonitrile, 49.95% isopropyl alcohol. ESI/MS/MS was performed online using ESI/MS/MS in the positive multiple-reaction monitoring mode. Sphingomyelins and phosphatidylcholines were quantified as the ratio of analyte to internal standard. The following compounds were used as internal standards: 19:0–19:0 PC and 12:0 SM.
Recombinant SMS1 and SMS2 preparation
The cDNAs for human SMS1 (UniProt ID Q86VZ5) and SMS2 (UniProt ID Q8NHU3) cDNAs were cloned into an expression vector modified from pFastBac Dual, with a Strep tag II amino acid sequence (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys) and a PreScission Protease recognition sequence added to the N terminus of cDNA of SMS1 or SMS2 for affinity chromatography. The SMS protein was expressed using a baculovirus/Spodoptera frugiperda (SF9) cell system and purified with the following procedure. The SMS-expressing cells were harvested 60 h postinfection and resuspended in hypotonic buffer (10 mm NaCl, 10 mm HEPES, pH 7.5). The cells were then lysed by centrifugation at 45,000 × g for 30 min at 4 °C, and the pellet was resuspended into hypertonic wash buffer (1 m NaCl, 25 mm HEPES, pH 7.5) supplemented with 0.1 mg/ml DNase, 5 mm MgCl2, and 1× protease inhibitor mixture, followed by homogenizing with a Dounce homogenizer. After centrifugation at 45,000 × g for 45 min at 4 °C, the pellet was resuspended into lysis buffer (150 mm NaCl, 20 mm HEPES, pH 7.5, 10% glycerol) supplemented with 0.1 mg/ml DNase, 5 mm MgCl2, and 1× protease inhibitor mixture and homogenized with a Dounce homogenizer. The membrane proteins were extracted from membrane by adding n-dodecyl-β-d-maltopyranoside to a final concentration of 30 mm and gently swirling for 2–4 h at 4 °C. The solubilized mixture was then cleaned up by centrifugation, and the supernatant was subjected to affinity chromatography using Strep-Tactin® resin. The eluate containing SMS proteins was further isolated with size-exclusion chromatography using Superdex 200 10/300 GL in a mobile phase consisting of 150 mm NaCl, 20 mm HEPES, pH 7.5, and 1 mm n-dodecyl-β-d-maltopyranoside. The fractions were pooled and stored at −80 °C for the functional assay.
2OHOA in vivo study
C57BL/6 mice were treated with 2OHOA (600 mg/kg, intragastrically) once daily for 10 days. Fasting blood was collected, and cholesterol, PC, SM, and triglyceride were measured. The liver and small intestine homogenates were prepared, and SMS activity and LPCAT activity were performed accordingly.
Statistical analysis
Results are expressed as mean ± S.D. The statistical significance of the difference between two data means was determined with a two-tailed exact Mann–Whitney test, and differences among multiple groups were assessed by one-way analysis of variance followed by the Student–Newman–Keuls test. A difference for which p was <0.05 was considered statistically significant.
Author contributions
B. L., Q. L., J. H., I. K., and P. L. data curation; B. L., H. H. B., and K. R. formal analysis; B. L., T. D., J. D., D. Y., Y. Cao, and X.-C. J. validation; B. L., Q. L., J. H., I. K., P. L., M. M., Y. Chen, and Y. Cao investigation; B. L., Q. L., J. H., I. K., P. L., M. M., Y. Chen, H. H. B., K. R., and Y. Cao methodology; T. D., J. D., D. Y., and X.-C. J. supervision; Y. Cao and X.-C. J. conceptualization; Y. Cao and X.-C. J. writing-review and editing; X.-C. J. funding acquisition; X.-C. J. visualization; X.-C. J. writing-original draft.
This work was supported by National Institutes of Health Grant 1R01 HL139582-01A1 and Veterans Affairs Merit Grant 000900-01 (to X.-C. J.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- 2OHOA
- 2-hydroxy-oleic acid
- PC
- phosphatidylcholine
- SM
- sphingomyelin
- SMS
- sphingomyelin synthase
- rSMS1 and rSMS2
- recombinant SMS1 and SMS2, respectively
- LPCAT
- lysophosphatidylcholine acyltransferase
- NBD
- 12-(N-methyl-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl))
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PMSF
- phenylmethylsulfonyl fluoride
- TLC
- thin-layer chromatography
- ESI
- electrospray ionization.
References
- 1. Martínez J., Gutiérrez A., Casas J., Lladó V., López-Bellan A., Besalduch J., Dopazo A., and Escribá P. V. (2005) The repression of E2F-1 is critical for the activity of Minerval against cancer. J. Pharmacol. Exp. Ther. 315, 466–474 10.1124/jpet.105.088716 [DOI] [PubMed] [Google Scholar]
- 2. Terés S., Lladó V., Higuera M., Barceló-Coblijn G., Martin M. L., Noguera-Salvà M. A., Marcilla-Etxenike A., García-Verdugo J. M., Soriano-Navarro M., Saus C., Gómez-Pinedo U., Busquets X., and Escribá P. V. (2012) 2-Hydroxyoleate, a nontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc. Natl. Acad. Sci. U.S.A. 109, 8489–8494 10.1073/pnas.1118349109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Llado V., Gutierrez A., Martínez J., Casas J., Terés S., Higuera M., Galmés A., Saus C., Besalduch J., Busquets X., and Escribá P. V. (2010) Minerval induces apoptosis in Jurkat and other cancer cells. J. Cell. Mol. Med. 14, 659–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lladó V., Terés S., Higuera M., Alvarez R., Noguera-Salva M. A., Halver J. E., Escribá P. V., and Busquets X. (2009) Pivotal role of dihydrofolate reductase knockdown in the anticancer activity of 2-hydroxyoleic acid. Proc. Natl. Acad. Sci. U.S.A. 106, 13754–13758 10.1073/pnas.0907300106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martínez J., Vögler O., Casas J., Barceló F., Alemany R., Prades J., Nagy T., Baamonde C., Kasprzyk P. G., Terés S., Saus C., and Escribá P. V. (2005) Membrane structure modulation, protein kinase C alpha activation, and anticancer activity of minerval. Mol. Pharmacol. 67, 531–540 [DOI] [PubMed] [Google Scholar]
- 6. Barceló-Coblijn G., Martin M. L., de Almeida R. F., Noguera-Salvà M. A., Marcilla-Etxenike A., Guardiola-Serrano F., Lüth A., Kleuser B., Halver J. E., and Escribá P. V. (2011) Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc. Natl. Acad. Sci. U.S.A. 108, 19569–19574 10.1073/pnas.1115484108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merrill A. H. Jr., and Jones D. D. (1990) An update of the enzymology and regulation of sphingomyelin metabolism. Biochim. Biophys. Acta 1044, 1–12 10.1016/0005-2760(90)90211-F [DOI] [PubMed] [Google Scholar]
- 8. Huitema K., van den Dikkenberg J., Brouwers J. F., and Holthuis J. C. (2004) Identification of a family of animal sphingomyelin synthases. EMBO J. 23, 33–44 10.1038/sj.emboj.7600034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamaoka S., Miyaji M., Kitano T., Umehara H., and Okazaki T. (2004) Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J. Biol. Chem. 279, 18688–18693 10.1074/jbc.M401205200 [DOI] [PubMed] [Google Scholar]
- 10. Vacaru A. M., Tafesse F. G., Ternes P., Kondylis V., Hermansson M., Brouwers J. F., Somerharju P., Rabouille C., and Holthuis J. C. (2009) Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J. Cell Biol. 185, 1013–1027 10.1083/jcb.200903152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yano M., Watanabe K., Yamamoto T., Ikeda K., Senokuchi T., Lu M., Kadomatsu T., Tsukano H., Ikawa M., Okabe M., Yamaoka S., Okazaki T., Umehara H., Gotoh T., Song W. J., Node K., Taguchi R., Yamagata K., and Oike Y. (2011) Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J. Biol. Chem. 286, 3992–4002 10.1074/jbc.M110.179176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Z., Fan Y., Liu J., Li Y., Huan C., Bui H. H., Kuo M. S., Park T. S., Cao G., and Jiang X. C. (2012) Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 32, 1577–1584 10.1161/ATVBAHA.112.251538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J., Zhang H., Li Z., Hailemariam T. K., Chakraborty M., Jiang K., Qiu D., Bui H. H., Peake D. A., Kuo M. S., Wadgaonkar R., Cao G., and Jiang X. C. (2009) Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler. Thromb. Vasc. Biol. 29, 850–856 10.1161/ATVBAHA.109.185223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitsutake S., Zama K., Yokota H., Yoshida T., Tanaka M., Mitsui M., Ikawa M., Okabe M., Tanaka Y., Yamashita T., Takemoto H., Okazaki T., Watanabe K., and Igarashi Y. (2011) Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 286, 28544–28555 10.1074/jbc.M111.255646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugimoto M., Shimizu Y., Zhao S., Ukon N., Nishijima K., Wakabayashi M., Yoshioka T., Higashino K., Numata Y., Okuda T., Tamaki N., Hanamatsu H., Igarashi Y., and Kuge Y. (2016) Characterization of the role of sphingomyelin synthase 2 in glucose metabolism in whole-body and peripheral tissues in mice. Biochim. Biophys. Acta 1861, 688–702 10.1016/j.bbalip.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 16. Sakamoto H., Yoshida T., Sanaki T., Shigaki S., Morita H., Oyama M., Mitsui M., Tanaka Y., Nakano T., Mitsutake S., Igarashi Y., and Takemoto H. (2017) Possible roles of long-chain sphingomyelines and sphingomyelin synthase 2 in mouse macrophage inflammatory response. Biochem. Biophys. Res. Commun. 482, 202–207 10.1016/j.bbrc.2016.11.041 [DOI] [PubMed] [Google Scholar]
- 17. Fan Y., Shi F., Liu J., Dong J., Bui H. H., Peake D. A., Kuo M. S., Cao G., and Jiang X. C. (2010) Selective reduction in the sphingomyelin content of atherogenic lipoproteins inhibits their retention in murine aortas and the subsequent development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 2114–2120 10.1161/ATVBAHA.110.213363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Z., Zhang H., Liu J., Liang C. P., Li Y., Li Y., Teitelman G., Beyer T., Bui H. H., Peake D. A., Zhang Y., Sanders P. E., Kuo M. S., Park T. S., Cao G., and Jiang X. C. (2011) Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol. Cell. Biol. 31, 4205–4218 10.1128/MCB.05893-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lou B., Dong J., Li Y., Ding T., Bi T., Li Y., Deng X., Ye D., and Jiang X. C. (2014) Pharmacologic inhibition of sphingomyelin synthase (SMS) activity reduces apolipoprotein-B secretion from hepatocytes and attenuates endotoxin-mediated macrophage inflammation. PLoS One 9, e102641 10.1371/journal.pone.0102641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Y., Dong J., Ding T., Kuo M. S., Cao G., Jiang X. C., and Li Z. (2013) Sphingomyelin synthase 2 activity and liver steatosis: an effect of ceramide-mediated peroxisome proliferator-activated receptor γ2 suppression. Arterioscler. Thromb. Vasc. Biol. 33, 1513–1520 10.1161/ATVBAHA.113.301498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gándola Y. B., Pérez S. E., Irene P. E., Sotelo A. I., Miquet J. G., Corradi G. R., Carlucci A. M., and Gonzalez L. (2014) Mitogenic effects of phosphatidylcholine nanoparticles on MCF-7 breast cancer cells. Biomed. Res. Int. 2014, 687037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Y., Chen Y. Q., Bonacci T. M., Bredt D. S., Li S., Bensch W. R., Moller D. E., Kowala M., Konrad R. J., and Cao G. (2008) Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J. Biol. Chem. 283, 8258–8265 10.1074/jbc.M710422200 [DOI] [PubMed] [Google Scholar]
- 23. Ishitsuka R., Yamaji-Hasegawa A., Makino A., Hirabayashi Y., and Kobayashi T. (2004) A lipid-specific toxin reveals heterogeneity of sphingomyelin-containing membranes. Biophys. J. 86, 296–307 10.1016/S0006-3495(04)74105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J., Huan C., Chakraborty M., Zhang H., Lu D., Kuo M. S., Cao G., and Jiang X. C. (2009) Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ. Res. 105, 295–303 10.1161/CIRCRESAHA.109.194613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hailemariam T. K., Huan C., Liu J., Li Z., Roman C., Kalbfeisch M., Bui H. H., Peake D. A., Kuo M. S., Cao G., Wadgaonkar R., and Jiang X. C. (2008) Sphingomyelin synthase 2 deficiency attenuates NFκB activation. Arterioscler. Thromb. Vasc. Biol. 28, 1519–1526 10.1161/ATVBAHA.108.168682 [DOI] [PubMed] [Google Scholar]
- 26. Fernández R., Garate J., Lage S., Terés S., Higuera M., Bestard-Escalas J., Martin M. L., López D. H., Guardiola-Serrano F., Escribá P. V., Barceló-Coblijn G., and Fernández J. A. (2016) Optimized protocol to analyze changes in the lipidome of xenografts after treatment with 2-hydroxyoleic acid. Anal. Chem. 88, 1022–1029 10.1021/acs.analchem.5b03978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kennedy E. P., and Weiss S. B. (1956) The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 222, 193–214 [PubMed] [Google Scholar]
- 28. Lands W. E. (1958) Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J. Biol. Chem. 231, 883–888 [PubMed] [Google Scholar]
- 29. Ma Z., Wang X., Nowatzke W., Ramanadham S., and Turk J. (1999) Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J. Biol. Chem. 274, 9607–9616 10.1074/jbc.274.14.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen X., Hyatt B. A., Mucenski M. L., Mason R. J., and Shannon J. M. (2006) Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc. Natl. Acad. Sci. U.S.A. 103, 11724–11729 10.1073/pnas.0604946103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakanishi H., Shindou H., Hishikawa D., Harayama T., Ogasawara R., Suwabe A., Taguchi R., and Shimizu T. (2006) Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1): expression in alveolar type II cells and possible involvement in surfactant production. J. Biol. Chem. 281, 20140–20147 10.1074/jbc.M600225200 [DOI] [PubMed] [Google Scholar]
- 32. Shindou H., Hishikawa D., Nakanishi H., Harayama T., Ishii S., Taguchi R., and Shimizu T. (2007) A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells: cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J. Biol. Chem. 282, 6532–6539 10.1074/jbc.M609641200 [DOI] [PubMed] [Google Scholar]
- 33. Hishikawa D., Shindou H., Kobayashi S., Nakanishi H., Taguchi R., and Shimizu T. (2008) Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc. Natl. Acad. Sci. U.S.A. 105, 2830–2835 10.1073/pnas.0712245105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rong X., Albert C. J., Hong C., Duerr M. A., Chamberlain B. T., Tarling E. J., Ito A., Gao J., Wang B., Edwards P. A., Jung M. E., Ford D. A., and Tontonoz P. (2013) LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 18, 685–697 10.1016/j.cmet.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dobrzyńska I., Szachowicz-Petelska B., Darewicz B., and Figaszewski Z. A. (2015) Characterization of human bladder cell membrane during cancer transformation. J. Membr. Biol. 248, 301–307 10.1007/s00232-015-9770-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dobrzyńska I., Szachowicz-Petelska B., Sulkowski S., and Figaszewski Z. (2005) Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol. Cell. Biochem. 276, 113–119 10.1007/s11010-005-3557-3 [DOI] [PubMed] [Google Scholar]
- 37. Szachowicz-Petelska B., Dobrzyńska I., Skrodzka M., Darewicz B., Figaszewski Z. A., and Kudelski J. (2013) Phospholipid composition and electric charge in healthy and cancerous parts of human kidneys. J. Membr. Biol. 246, 421–425 10.1007/s00232-013-9554-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakai K., Okuyama H., Yura J., Takeyama H., Shinagawa N., Tsuruga N., Kato K., Miura K., Kawase K., and Tsujimura T. (1992) Composition and turnover of phospholipids and neutral lipids in human breast cancer and reference tissues. Carcinogenesis 13, 579–584 10.1093/carcin/13.4.579 [DOI] [PubMed] [Google Scholar]
- 39. Ridgway N. D. (2013) The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. Biol. 48, 20–38 10.3109/10409238.2012.735643 [DOI] [PubMed] [Google Scholar]
- 40. Cheng M., Bhujwalla Z. M., and Glunde K. (2016) Targeting Phospholipid Metabolism in Cancer. Front. Oncol. 6, 266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vance D. E. (2014) Phospholipid methylation in mammals: from biochemistry to physiological function. Biochim. Biophys. Acta 1838, 1477–1487 10.1016/j.bbamem.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 42. Martin M. L., Barceló-Coblijn G., de Almeida R. F., Noguera-Salvà M. A., Terés S., Higuera M., Liebisch G., Schmitz G., Busquets X., and Escribá P. V. (2013) The role of membrane fatty acid remodeling in the antitumor mechanism of action of 2-hydroxyoleic acid. Biochim. Biophys. Acta 1828, 1405–1413 10.1016/j.bbamem.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 43. Ibarguren M., López D. J., Encinar J. A., González-Ros J. M., Busquets X., and Escribá P. V. (2013) Partitioning of liquid-ordered/liquid-disordered membrane microdomains induced by the fluidifying effect of 2-hydroxylated fatty acid derivatives. Biochim. Biophys. Acta 1828, 2553–2563 10.1016/j.bbamem.2013.06.014 [DOI] [PubMed] [Google Scholar]