Figure 1.
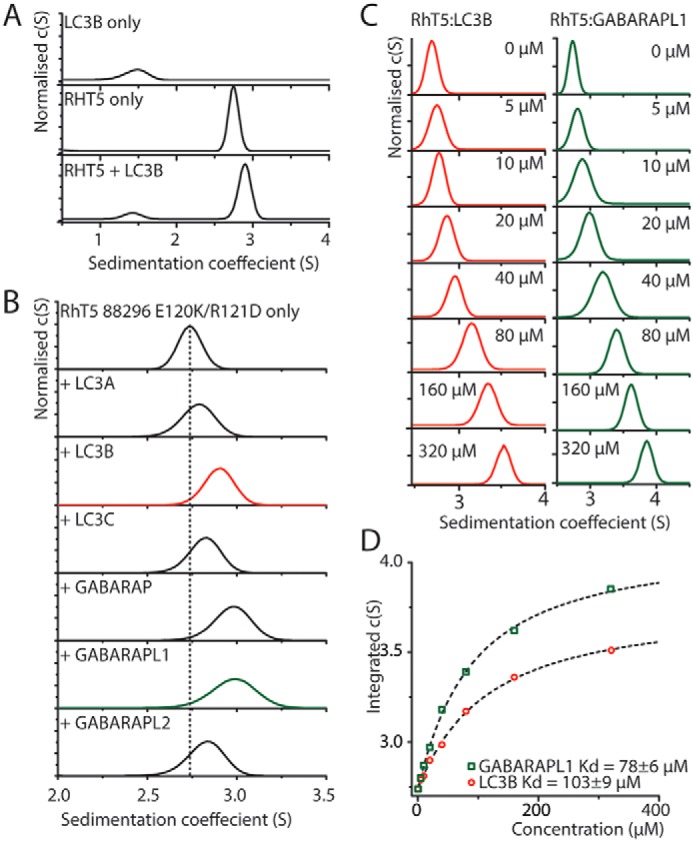
SV–AUC analysis shows that purified Trim5α binds to six isoforms of mATG8s. A, c(s) analysis of SV–AUC of 20 μm LC3B, RhT5 88–296 EK/RD, or an equimolar mixture. B, c(s) analysis of 20 μm of RhT5 88–296 EK/RD and equimolar concentration of the six mATG8 isoforms. C, c(s) analysis of 20 μm RhT5 88–296 EK/RD and increasing concentrations (0–320 μm) of either LC3B or GABARAPL1. D, peak centroid position derived from integration of the c(s) function from C versus LC3B or GABARAPL1 concentration. A one-site binding model has been used to determine the equilibrium dissociation constant (dashed lines).
