Table 3.
Molecular descriptors obtained for the analyzed compounds (aryl or heteroaryl monocyclic derivatives) a.
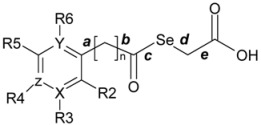 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | X | Y | Z | n | R2 | R3 | R4 | R5 | R6 | AlogP | Volb | μ (D)c | IC50(μM)e |
| 1 | C | C | C | 0 | H | H | H | H | H | 1.795 | 68.58 | 3.465 | 6.8 |
| 2 | C | C | C | 0 | H | H | CN | H | H | 1.673 | 82.16 | 2.865 | 10.0 |
| 3 | C | C | C | 0 | H | H | CF3 | H | H | 2.737 | 92.64 | 1.137 | NE |
| 4 | C | C | C | 0 | H | H | Cl | H | H | 2.459 | 83.19 | 2.628 | NE |
| 5 | C | C | C | 0 | H | H | CH3 | H | H | 2.281 | 82.76 | 4.430 | NE |
| 6 | C | C | C | 0 | H | H | C(CH3)3 | H | H | 3.195 | 125.55 | 4.584 | NE |
| 7 | C | C | C | 0 | H | H | OCH3 | H | H | 1.778 | 89.51 | 5.337 | NE |
| 8 | C | C | C | 0 | Cl | H | H | H | H | 2.459 | 82.97 | 2.874 | NE |
| 9 | C | C | C | 0 | Br | H | H | H | H | 2.543 | 91.02 | 4.032 | NE |
| 10 | C | C | C | 0 | I | H | H | H | H | 2.373 | 100.49 | 3.259 | NE |
| 11 | C | C | C | 1 | H | H | H | H | H | 1.829 | 82.73 | 3.418 | 2.9 |
| Ref. | X | Y | Z | n | R2 | R3 | R4 | R5 | R6 | AlogP | Volb | μ ( D)c | IC50( μ M)d |
| 12 | C | C | C | 2 | H | H | H | H | H | 2.286 | 93.29 | 4.243 | NE |
| 13 | C | C | C | 0 | H | Cl | H | Cl | H | 3.123 | 98.15 | 2.441 | NE |
| 14 | C | C | C | 0 | H | OCH3 | H | OCH3 | H | 1.762 | 111.05 | 4.591 | 4.0 |
| 15 | C | C | C | 0 | H | OCH3 | OCH3 | OCH3 | H | 1.745 | 131.49 | 5.090 | NE |
| 16 | C | C | C | 0 | H | -O-CH2-O- | H | H | 1.563 | 88.78 | 4.352 | NE | |
| 19 | C | C | N | 0 | H | H | - | H | H | 0.644 | 64.45 | 1.081 | NE |
| 20 | N | C | C | 0 | H | H | H | H | H | 0.644 | 64.64 | 3.543 | NE |
| 21 | N | C | C | 0 | Cl | H | H | H | H | 1.518 | 78.98 | 4.094 | 10.0 |
| 22 | N | C | C | 0 | S(CH2)2CH3 | H | H | H | H | 2.597 | 126.66 | 4.964 | NE |
| 23 | 2-thienyl | 1.520 | 64.02 | 3.315 | NE | ||||||||
| 24 | N | N | C | 0 | H | H | H | H | - | -0.078 | 60.35 | 3.096 | NE |
| MSA | - | - | - | - | - | - | - | - | - | - | - | - | 8.38 |
| Etoposide | - | - | - | - | - | - | - | - | - | - | - | - | 13.6 ± 2.2 |
a General structure for the analysed compounds showing the bonds (a–e) selected for the conformational analysis. b Volume (average value obtained from the lowest energy conformations) of the cyclic fragment in Å 3. c Dipolar moment (in Debyes) calculated for the representative low-energy. d Cytotoxic activity in PC-3 cell line, NE= no effect.
