Table 4.
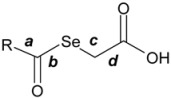
Molecular descriptors obtained for the analyzed compounds (aryl or heteroaryl bicyclic derivatives) a.
 | |||||
|---|---|---|---|---|---|
| Ref. | R | AlogP | Volb | μ (D)c | IC50(μM)d |
| 17 | naphthyl | 2.703 | 106.65 | 3.759 | NE |
| 18 | diphenylmethyl | 3.324 | 144.34 | 3.632 | NE |
| 25 | 2-quinolyl | 2.409 | 102.41 | 4.451 | NE |
| 26 | 3-quinolyl | 1.981 | 102.22 | 2.685 | NE |
| MSA | - | - | - | - | 8.38 |
| Etoposide | - | - | - | - | 13.6 ± 2.2 |
a General structure for the analysed compounds showing the bonds (a–d) selected for the conformational analysis. b Volume (average value obtained from the lowest energy conformations) of the cyclic fragment in Å 3. c Dipolar moment (in Debyes) calculated for the representative low-energy conformation. d Cytotoxic activity in PC-3 cell line, NE= no effect.
