Table 5.
Mechano-quantic descriptive parameters (semiempirical: PM6) obtained for the analyzed compounds (aryl or heteroaryl monocyclic derivatives).
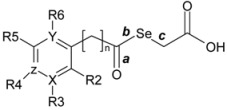 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | X | Y | Z | n | R2 | R3 | R4 | R5 | R6 | pKab | Bond order | HOMOc | LUMOd | EL-M | Q_See | IC50 | ||
| a | b | c | (M)f | |||||||||||||||
| 1 | C | C | C | 0 | H | H | H | H | H | 2,119 | 1,8315 | 0,9068 | 0,9618 | -9,210 | -1,552 | 7,658 | 0.0577 | 6.8 |
| 2 | C | C | C | 0 | H | H | CN | H | H | 2,057 | 1,8478 | 0,9050 | 0,9608 | -9,422 | -2,022 | 7,400 | 0.0778 | 10.0 |
| 3 | C | C | C | 0 | H | H | CF3 | H | H | 2,148 | 1,8492 | 0,9048 | 0,9608 | -9,399 | -1,894 | 7,505 | -0.0303 | NE |
| 4 | C | C | C | 0 | H | H | Cl | H | H | 2,188 | 1,7914 | 0,9659 | 0,9624 | -9,382 | -1,666 | 7,716 | -0.0394 | NE |
| 5 | C | C | C | 0 | H | H | CH3 | H | H | 2,184 | 1,8230 | 0,9037 | 0,9613 | -9,002 | -1,487 | 7,515 | 0.0491 | NE |
| 6 | C | C | C | 0 | H | H | C(CH3)3 | H | H | 2,182 | 1,8256 | 0,9027 | 0,9611 | -9,108 | -1,403 | 7,705 | 0.0485 | NE |
| 7 | C | C | C | 0 | H | H | OCH3 | H | H | 2,196 | 1,8159 | 0,8940 | 0,9592 | -9,039 | -1,208 | 7,831 | 0.0433 | NE |
| 8 | C | C | C | 0 | Cl | H | H | H | H | 2,360 | 1,8508 | 0,9320 | 0,9673 | -9,258 | -1,384 | 7,874 | 0.0292 | NE |
| 9 | C | C | C | 0 | Br | H | H | H | H | 2,346 | 1,8611 | 0,9172 | 0,9524 | -9,295 | -1,244 | 8,051 | 0.0134 | NE |
| 10 | C | C | C | 0 | I | H | H | H | H | 2,388 | 1,8721 | 0,9069 | 0,9626 | -8,864 | -1,582 | 7,282 | -0.0182 | NE |
| 11 | C | C | C | 1 | H | H | H | H | H | 2,370 | 1,8419 | 0,9742 | 0,9841 | -9,439 | -0,936 | 8,503 | 0.0681 | 2.9 |
| 12 | C | C | C | 2 | H | H | H | H | H | 2,378 | 1,8360 | 0,9799 | 0,9622 | -9,485 | -1,138 | 8,347 | 0.0580 | NE |
| 13 | C | C | C | 0 | H | Cl | H | Cl | H | 1,858 | 1,8482 | 0,9123 | 0,9626 | -9,410 | -1,961 | 7,449 | -0.0331 | NE |
| 14 | C | C | C | 0 | H | OCH3 | H | OCH3 | H | 2,791 | 1,9205 | 0,9126 | 0,9673 | -9,275 | -1,063 | 8,212 | 0.0693 | 4.0 |
| 15 | C | C | C | 0 | H | OCH3 | OCH3 | OCH3 | H | 2,431 | 1,8021 | 0,9528 | 0,9633 | -8,770 | -1,329 | 7,441 | 0.0652 | NE |
| 16 | C | C | C | 0 | H | -OCH2O- | H | H | 2,083 | 1,8413 | 0,9068 | 0,9620 | -9,223 | -1,630 | 7,593 | 0.0720 | NE | |
| 19 | C | C | N | 0 | H | H | - | H | H | 1,985 | 1,7891 | 0,9918 | 0,9766 | -9,546 | -1,717 | 7,829 | 0.0825 | NE |
| 20 | N | C | C | 0 | H | H | H | H | H | 2,230 | 1,7775 | 0,9840 | 0,9767 | -9,483 | -1,663 | 7,820 | 0.0712 | NE |
| 21 | N | C | C | 0 | Cl | H | H | H | H | 2,145 | 1,8279 | 0,9540 | 0,9686 | -9,368 | -1,712 | 7,656 | 0.0129 | 10.0 |
| 22 | N | C | C | 0 | S(CH2)2CH3 | H | H | H | H | 2,486 | 1,8722 | 0,8969 | 0,9628 | -8,871 | -1,585 | 7,286 | -0.0093 | NE |
| 23 | 2-thienyl | 2,181 | 1,7872 | 0,9417 | 0,9598 | -9,204 | -1,696 | 7,508 | -0.0438 | NE | ||||||||
| 24 | N | N | C | 0 | H | H | H | H | - | 1,813 | 1,7829 | 1,0108 | 0,9746 | -9,406 | -2,030 | 7,376 | 0.1126 | NE |
