Abstract
Background
The association between diabetes mellitus (DM) treatment and dementia is not well understood.
Objective
To investigate the association between treatment of diabetes, hypoglycemia, and dementia risk.
Research design and methods
We performed a systematic review and meta-analysis of pharmacological treatment of diabetes and incident or progressive cognitive impairment. We searched Ovid MEDLINE, Embase, Cochrane Central Registry of Controlled Trials, and PsychINFO from inception to 18 October 2017. We included cross-sectional, case–control, cohort, and randomized controlled studies. The study was registered with PROSPERO (ID CRD42017077953).
Results
We included 37 studies into our systematic review and 13 into our meta-analysis. Ten studies investigated any antidiabetic treatment compared with no treatment or as add-on therapy to prior care. Treatment with an antidiabetic agent, in general, was not associated with incident dementia (risk ratio (RR) 1.01; 95% CI 0.93 to 1.10). However, we found differential effects across drug classes, with a signal of harm associated with insulin therapy (RR 1.21; 95% CI 1.06 to 1.39), but potentially protective effects with thiazolidinedione exposure (RR 0.71; 95% CI 0.55 to 0.93). Severe hypoglycemic episodes were associated with a nearly twofold increased likelihood of incident dementia (RR 1.77; 95% CI 1.35 to 2.33). Most studies did not account for DM duration or severity.
Conclusions and limitations
The association between treatment for diabetes and dementia is differential according to drug class, which is potentially mediated by hypoglycemic risk. Not accounting for DM duration and/or severity is a major limitation in the available evidence base.
Keywords: diabetes mellitus, treatment, dementia, mild cognitive impairment
Significance of this study.
What is already known about this subject?
Diabetes is a risk factor for developing dementia, yet the impact of diabetes management on cognitive decline remains uncertain.
What are the new findings?
We found that antidiabetic treatment effects on cognitive outcomes may differ by drug class.
We also found that severe hypoglycemia is associated with nearly a twofold increased risk of incident dementia.
How might these results change the focus of research or clinical practice?
Past studies have poorly accounted for duration and severity of diabetes, which introduces confounding in the association between specific antidiabetic treatments and incident cognitive impairment. This is an area that may be addressed by future studies.
Introduction
Approximately 10% of people with dementia have diabetes mellitus (DM).1 Diabetes is a risk factor for developing vascular dementia (VaD),2 3 mixed dementia, Alzheimer’s disease (AD), and mild cognitive impairment (MCI).4 5 Dementia in patients with DM occurs at a younger age and is more frequently vascular in etiology compared with individuals without DM.6
Studies suggest that cognitive decline in older individuals with DM is associated with poor glycemic control and more frequent episodes of severe hypoglycemia.7 However, the impact of diabetes management on the rate of cognitive decline in individuals with established cognitive deficits including dementia remains uncertain.7 The association between treatment of DM and cognitive outcomes has been variably reported across studies with significant differences in the type of diabetic treatment and its intensity, size of the studies, follow-up duration, and handling of potential confounding. Addressing this, we conducted a systematic review and meta-analysis to investigate the association between pharmacological treatment of DM and cognitive outcomes in adults.
Methods
Our primary objective was to determine whether specific pharmacological treatments for DM (compared with placebo or an alternative agent) were associated with cognitive outcomes in adults (≥18 years of age) with diabetes. As a secondary objective, we examined the association between the frequency of severe hypoglycemia and adverse cognitive outcomes.7 The study was registered with PROSPERO (ID number CRD42017077953) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.8
Search strategy and literature sources
We performed literature searches of Ovid MEDLINE, Embase, Cochrane Central Registry of Controlled Trials, and PsychINFO electronic databases from inception to 18 October 2017. Two authors (JMM and BSM) reviewed reference lists of included articles for additional relevant studies and contacted study authors of included studies if further information was required.
The main search concepts were type 2 DM, dementia or mild cognitive impairment, and pharmacological agents used to treat DM (online supplementary appendix). We developed a comprehensive list of pharmacological agents in use for the treatment of DM based on numerous international clinical practice guidelines including the Canadian Diabetes Association, the American Diabetes Association, Diabetes Australia, and the National Institute for Health and Care Excellence. We sought the expertise of a content expert in the management of DM to assist in creating the list of pharmacological treatments. The search strategy was developed in consultation with two medical librarians at the University of Calgary. Within each search cluster, the keywords Medical Subject Headings, EMTREE, PsychINFO, and Cochrane terms were combined using “or”. Each cluster was then combined using “and” (online supplementary appendix).
bmjdrc-2018-000563supp001.docx (1.8MB, docx)
Study screening and selection
Two independent reviewers (BSM and JMM) screened titles and abstracts in duplicate. The primary inclusion criterion was pharmacological treatment of DM in adults. In the initial screening phase, titles and abstracts were included if the study enrolled participants with DM and any form of cognitive outcome. In the full-text screening phase, articles were reviewed in duplicate (BSM and JMM) and included if the study investigated the pharmacological treatment of DM in adults with both DM and cognitive outcome measures. Studies were excluded if they did not enroll patients with both DM and dementia, investigate one or more pharmacological treatments for DM, report cognitive outcomes, present primary data, or if they were duplicate reports or reported findings of another study. Discrepancies were resolved by consensus.
Data extraction, synthesis, and analysis
A kappa statistic was calculated to quantify agreement on the selection of papers for full-text review. This was done using inter-rater agreement and possibility of agreement due to chance, calculated in Stata V.14.2.426. Data from studies selected for full-text review were extracted in duplicate for quality assessment and analysis. Data collection included (if available) author; year of publication; country of origin; study design; population demographics (mean or median age, percentage female); method of diagnosis for DM, dementia, and/or MCI; intervention/exposure and control group; study duration; number of participants enrolled in study; number of participants completing study; duration of DM; glycated hemoglobin A1c (A1c) values; hypoglycemic episodes; and cognitive outcomes. Only studies using validated instruments to assess cognition (eg, Mini-Mental State Examination (MMSE), Alzheimer’s Disease Assessment Scale–Cognitive Subscale) were eligible for inclusion.
The primary composite outcome of interest was the risk of incident dementia or progression of cognitive impairment associated with pharmacological treatment of DM. We additionally examined cognitive outcomes (either incident dementia, or progression of dementia or MCI) associated with the frequency of severe hypoglycemic episodes.
Quality and risk of bias assessment
Quality and risk of bias assessments were performed in duplicate. We used the Newcastle Ottawa Quality Assessment Scale for non-randomized studies (cohort, cross-sectional, and case–control studies).9 For randomized controlled trials, the Cochrane Collaboration’s tool for assessing risk of bias in randomized studies was used.10
Meta-analysis of relative risk
We used risk ratios (RRs) as the common measure of association across studies. HRs were considered to be interchangeable with RRs as per methods described in previous meta-analysis by Chen et al.11 ORs were converted to RRs using the formula: RR=OR/[(1−P0)+(P0×OR)], where P0 is the incidence of the outcome of interest in the non-exposed (control) group. When there were insufficient data, study authors were contacted to request additional information if possible. A random-effects model was used to pool estimates across studies to account for both between-study and within-study variance. This was based on our assumption that there would be no one true estimate, but rather a distribution of estimates that would vary across studies. Where adequate data were available, we performed stratified analysis by mean age (<65 years vs ≥65 years), study size (≥10 000 participants versus <10 000), design (randomized controlled trials vs observational studies), study duration (≥3 years vs <3 years), and class of pharmacological agent(s). We divided sample sizes into <10 000 versus ≥10 000 individuals to ensure roughly equal numbers of studies were present in each stratified group. We categorized studies that investigated ≥1 antidiabetic medication compared with either no treatment or add-on to usual care as intensive treatment. We also evaluated the association between severe hypoglycemic episodes and the risk of adverse cognitive outcomes.
We assessed for between-study heterogeneity using the I2 test statistic. Low, moderate, and high heterogeneity were defined as <25%, 25%–50%, and >50%, respectively.12 Stratified analyses were performed to explore for potential sources of heterogeneity when present. We used STATA V.14.2 (College Station, Texas, USA) to perform statistical analysis.
Assessment of publication bias
Conventional methods to assess for publication bias, such as funnel plots, are challenging to interpret for meta-analyses of observational studies where small study effects may be present. The presence of asymmetry may not necessarily represent publication bias, but rather clinical heterogeneity or bias inherent to the included studies (eg, residual confounding). As such, we did not assess for publication bias because the majority of included studies were observational in design.
Results
The electronic database search produced 5088 unique citations (online supplementary figure). After screening titles and abstracts, 370 articles were identified for full-text review. Of these, 37 studies were included in our systematic review and 13 of these were incorporated into our quantitative (meta) analysis. There was overall excellent agreement between the reviewers on articles selected for full-text review (κ=0.81; 95% CI 0.71 to 0.91).
Of the 37 included studies, most were from Asia (n=14), Europe (n=10), and North America (n=10) and published between 1996 and 2017 (75% were published within the last 5 years; table 1). Study size ranged from 634 to 145 928 participants (72% were >1000 participants). Nearly one-third (27%) of studies did not report mean or median participant ages. When provided, 70% of studies reported a mean age >65. Duration of follow-up was reported in 81% of studies. This ranged from 6 months to 14.7 years (77% >3 years). Metformin and insulin were the two most common interventions. For most studies, the comparator was either placebo or standard care. Duration of DM, its severity and level of control, and frequency and severity of hypoglycemia were not consistently reported. All studies commented on the presence of dementia, but only a quarter (24%) provided data on MCI or dementia subtype (such as AD and VaD).
Table 1*.
Characteristics of included studies
| Author, year |
Study design | Study duration | Number of participants enrolled | Mean age (years) | Cognitive outcome | Cognitive diagnosis | Diabetes diagnosis | Intervention group |
Control group |
| Biemans et al,27
2015 |
Cohort | NR | 550 | 61.6 | Alzheimer’s disease (AD) and multi-infarct dementia | ICPC | ICPC | Metformin B12 deficient | Metformin B12 replete |
| Bruce et al, 23 2014 | Cohort | 14.7 years | 335 | 57.5 | Cognitive impairment (CI) and dementia | MMSE and CDR | Chart records | Insulin | NR |
| Cheng et al,29* 2014 |
Cohort | 3.1 years | 5420 | 73.6 | Dementia | ICD-9 | ICD-9 | Metformin, sulfonylurea, thiazolidinedione | NR |
| Chin et al,41* 2016 |
Cohort | 3.4 years | 1957 | 67.5 | Dementia | HIRAS claim database | HIRAS claim database | No hypoglycemic event, hypoglycemic event, two or more hypoglycemic events | NR |
| Chou et al,36* 2017 |
Case–control | 5 years | 19 203 | NR | Dementia | ICD-9 | ICD-9 | Pioglitazone (high cumulative dose, long-term use, high daily dose) | No pioglitazone |
| Cukierman et al,
2014 |
RCT | 6.2 years | 11 685 | 63.4 | CI | NR | NR | Insulin glargine targeting FBG <5.3 | Standard care |
| Fei et al,18* 2013 |
Cross-sectional | NR | 1109 | NR | All-cause dementia, AD, vascular dementia (VaD) | DSM-IV | WHO and ADA | Insulin | NR |
| Feinkohl et al,45
2014 |
Cohort | 4 years | 831 | 67.7 | NR | MMSE | NR | Hypoglycemic event | No hypoglycemia |
| Ha et al,
2017 |
Cohort | 10.5 years | 67 458 | NR | NR | NR | NR | Hypoglycemic event | NR |
| Heneka et al,20* 2015 |
Cohort | 6 years | 145 928 | NR | NR | NR | NR | Pioglitazones (≥8 calendar quarters and <8 calendar quarters), rosiglitazone, metformin, insulin | No pioglitazone, no piolitazone, no rosiglitazone, no metformin, no insulin |
| Hsiao et al,33
2014 |
Cohort | NR | 65 620 | NR | NR | NR | NR | Any metformin use, past metformin use, recent metformin use, current metformin use, cumulative use less than 2 years, cumulative use greater than 4 years | NR |
| Hsu et al,30* 2011 |
Cohort | 7 years | 25 393 | NR | Dementia | ICD-9 | NR | Sulfonylurea, metformin, sulfonylurea and metformin | NR |
| Huang et al,24
2014 |
Cohort | 10 years | 71 433 | 58.7 | AD | ICD-9 | ICD-9 | Metformin, sulfonylurea, thiazolidinedione, alpha glucosidase inhibitor, insulin | NR |
| Isik et al,37
2016 |
Cohort | 6 months | 253 | 75.4 | AD | NINDs criteria | Serum glucose | Sitagliptin | No sitagliptin, regular DM meds |
| Kuan et al,31* 2017 |
Cohort | 12 years | 9302 | 64.7 | Dementia, AD, VaD | ICD-9 | ICD-9 | Metformin | No metformin |
| Kuo et al,21* 2015 |
Cohort | 11 years | 33 709 | 62.3 | Dementia | ICD-9 | ICD-9 | Insulin use | No insulin use |
| Launer et al,
2011 |
RCT | 3.33 years | 2977 | 62.3 | NR | NR | NR | Intensive diabetic control target A1c <6%, standard glycemic control A1c target 7% to 7.9% | NR |
| Logroscino et al,17
2004 |
Cohort | 2 years | 1394 | 74.2 | Cognitive decline | Non-specified cognitive tests | Self-report | Oral antidiabetic agents, insulin, no antidiabetic treatment | NR |
| Ma et al,* 2015 |
Cohort | 4 years | 8213 | 75.3 | Mild cognitive impairment, dementia, VaD, other cause dementia | Petersen’s classification, NINCDS–ADRDA, DSM-III | Self-report, physician diagnosis of diabetes complication, medical records | Insulin, oral antidiabetic agent | No treatment |
| Mehta et al,43
2017 |
Cohort | 3.8 years | 53 055 | 75.5 | NR | NR | NR | NR | NR |
| Moore et al,28
2013 |
Cross-sectional | NR | 104 | 73.8 | NR | NR | NR | Metformin | No metformin |
| Murray et al, 2017 |
Cohort | 7 years | 1328 | 62.1 | NR | NR | NR | Intensive therapy (A1c<6%), standard therapy A1c 7–7.9% | NR |
| Naharci et al,34
2016 |
Cohort | NR | 1221 | 75.6 | Dementia | NR | NR | Metformin | No metformin |
| Ng et al,35
2014 |
Cohort | 4 years | 365 | 67 | NR | NR | NR | Metformin use <6 years, metformin use >6 years | NR |
| Orkaby et al,32
2017 |
Cohort | 5 years | 42 651 | 73.5 | Dementia, AD, VaD | ICD-9 | NR | Metformin, metformin, and sulfonylurea | Sulfonylurea |
| Ott et al,15* 1999 |
Cohort | 2.1 years | 6370 | 80.6 | Dementia, AD, VaD | DSM, NINCDS | NR | No drug, oral medication, insulin | NR |
| Ott et al,16
1996 |
Cross-sectional | NR | 6330 | 69.3 | AD and VaD | DSM and ADNI criteria Screen with MMSE or Geriatric Mental State Schedule followed by physician interview and brain imaging | Use of antidiabetic medication or blood glucose >11 | No drug, oral medication, insulin | NR |
| Parikh et al,13* 2011 |
Cohort | 2 years | 377 838 | 75.5 | Dementia | ICD-9-CM | ICD-9-CM | Insulin, oral antidiabetic agent | NR |
| Plastino et al,22
2010 |
Cohort | 1 year | 104 | 76.2 | AD | DSM-IV | Use of antidiabetic medication or blood glucose >11 | Oral agents only, insulin plus oral agents | NR |
| Rhee et al, 2014 |
Cohort | 3.4 years | 1957 | NR | NR | NR | NR | Hypoglycemic event | NR |
| Sato et al, 2011 |
RCT | 6 months | 42 | 77.4 | NR | NINCDS, CDR score 0.5 or 1 | DM drug Rx or elevated FBG | Pioglitazone in addition to use antidiabetic medications | No pioglitazone only regular antidiabetic medications |
| Trento et al, 2015 |
Cohort | 8 years | 498 | 66.8 | NR | NR | NR | Insulin | No insulin |
| Whitmer et al,25
2013 |
Cohort | 5 years | 14 891 | NR | NR | NR | NR | Metformin, sulfonylurea, thiazolidinedione, insulin | SU acted as reference |
| Whitmer et al,40* 2009 |
Cohort | 3.8 years | 16 667 | 66.3 | Dementia, AD, VaD | ICD-9 | Medical records, pharmacy Rx | One, two, or three episodes of severe hypoglycemia requiring hospital | NR |
| Yaffee et al,* 2013 |
Cohort | 12 years | 783 | 74.6 | NR | ICD-9 | Self-report, antidiabetic medication, elevated FBG according to ADA criteria | Hypoglycemic event | No hypoglycemic event |
| Yuan et al, 2015 |
Cohort | 2 years | NR | NR | NR | Self-report, diagnosis codes in Medicare claims, dementia drug use | NR | Metformin, thiazolidinedione, insulin | NR |
| Zullo et al,38
2017 |
Cohort | NR | 2016 | NR | NR | 1-point increase in MDS Cognitive Performance Scale score | NR | DPP-4 inhibitor | SU |
indicates the study was included within meta-analysis.
ADA, Alzheimer’s Disease Association; ADNI, Alzheimer’s Disease Neuroimaging Initiative; CDR, Clinical Dementia Rating; DPP-4, dipeptidyl peptidase-4; DSM, Diagnostic and Statistical Manual; FBG, fasting blood glucose; HIRAS, Health Insurance Review and Assessment Service; ICD, Internal Classification of Diseases; ICPC, International Classification of Primary Care; MDS, minimum data set; MMSE, Mini-Mental State Examination; NINCDS–ADRDA, National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association; NINDS, National Institute of Neurological Disorders and Stroke; NR, not reported; RCT, randomized controlled trial; SU, sulfonylurea.
Quality assessment of included studies
Thirty-three studies were observational (ie, cohort, cross-section, case–control studies) and four were randomized controlled trials (RCTs) (online supplementary tables 1 and 2).
Most observational studies (82%) enrolled exposed cases who were truly or somewhat representative of the population of interest. Nearly all studies (97%) enrolled non-exposed controls drawn from the same population as cases. When reported, the majority of studies (85%) had follow-up of ≥3 years. However, many studies did not provide information on the completeness of follow-up (eg, loss to follow-up was not reported in 64% of studies), how cognitive outcomes were assessed (39%), or whether the outcome of interest (eg, dementia) was present at enrolment (27%).
The study quality of the four RCTs was generally weak. Two did not provide sufficient detail to determine random sequence generation, allocation concealment, blinding of participants/personnel, or blinding of outcome assessment. Two were at risk of selective or incomplete outcome reporting. Two of the studies were potentially subject to industry bias.
Risk of incident dementia in DM
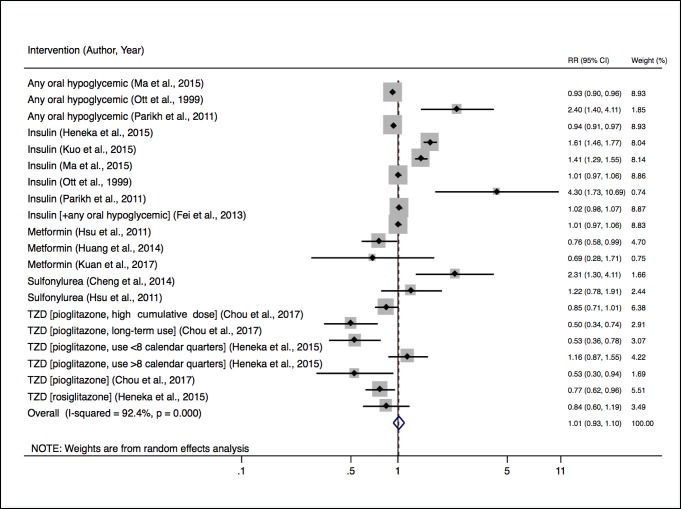
Thirteen studies were included in our meta-analysis. Ten investigated intensive therapy including any antidiabetic agent compared with no DM treatment, insulin added to prior therapy, metformin compared with no treatment, sulfonylurea compared with no treatment or as add-on therapy, and thiazolidinedione compared with no treatment or as add-on therapy. Intensive therapy (defined as add-on treatment) compared with prior care was not associated with incident dementia (RR 1.01; 95% CI 0.93 to 1.10). However, there was significant between-study heterogeneity (I2 92.4%, p=0.0001) (figure 1).
Figure 1.
Relative risk of developing dementia with intensive versus non-intensive antidiabetic treatment. RR, risk ratio; TZD, thiazolidinedione.
Meta-regression was performed to explore heterogeneity, evaluating the effect of intervention type, age, sample size, proportion loss to follow-up, and duration of study on cognitive outcomes (online supplementary table 3). Only stratification by age (<65 and ≥65) had a notable effect on the RR estimates. Younger compared with older individuals were at greater risk for adverse cognitive outcomes during treatment (1.66, 95% CI 1.05 to 2.61 (n=2) for <65; and 1.00, 95% CI 0.94 to 1.07 (n=7) for ≥65; p=0.046). The meta-regression analyses may have been underpowered to detect significant differences for the other variables assessed due to a limited number of studies within categories.
Risk estimates of dementia and other adverse cognitive outcomes by pharmacological agent
Any oral antidiabetic agent
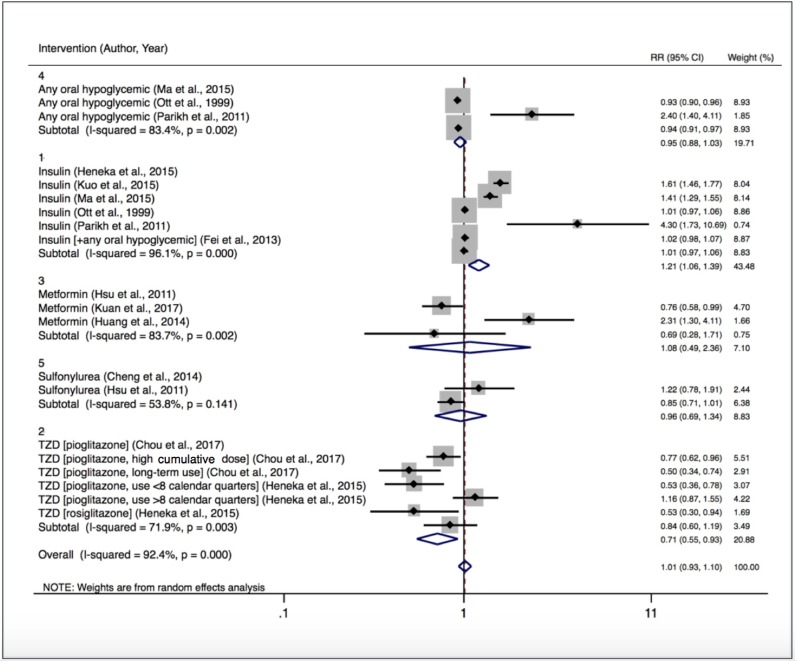
Heterogeneity remained high even after stratification by drug class (figure 2). Seven studies reported on the effect of any oral antidiabetic agent on cognitive outcomes.13–19 In our pooled analysis of unadjusted relative risks, when compared with usual care the use of an oral antidiabetic agent was not associated with a statistically significant difference in dementia incidence (RR 0.95; 95% CI 0.88 to 1.03).13–15
Figure 2.
Relative risk of developing dementia by treatment type. RR, risk ratio; TZD, thiazolidinedione.
Of the four studies not included in the meta-analysis, two reported ORs for incident dementia, but there were insufficient data to convert these into RRs.16 17 One reported a 23% reduced odds of cognitive decline with oral antidiabetic agents compared with no treatment that was not statistically significant (95% CI 0.54 to 1.08).17 The other reported no difference (OR 1.0; 95% CI 0.6 to 1.8).16
Insulin
Six studies were included in our meta-analysis.13–15 18 20 21 Treatment with insulin was associated with a 21% increased risk of incident dementia compared with other therapies or placebo (RR 1.21; 95% CI 1.06 to 1.39). In the eight studies that could not be included in our meta-analysis,16 17 19 22–26 there was a general pattern of adverse cognitive outcomes associated with insulin therapy, but there were important differences between the studies in patient characteristics, study duration, and whether (and how) DM duration and/or severity were adjusted for.
Metformin
Thirteen studies evaluated the association of metformin treatment on dementia in patients with DM20 24–35 with three included in our meta-analysis.24 30 31 A pooled estimate showed no significant difference in risk for developing dementia (RR 1.08; 95% CI 0.49 to 2.36). DM duration and severity could not be accounted for in the analysis.
In the studies not included in the meta-analysis, the effect of metformin was inconclusive. In a study that adjusted for DM duration, the odds of cognitive impairment among metformin users was reduced compared with non-users (OR 0.49; 95% CI 0.25 to 0.95). Long-term use (>6 years) was associated with the greatest cognitive benefit (OR 0.27; 95% CI 0.12 to 0.60).35 The variable results between studies were potentially related to inconsistent adjustment for DM duration and severity. Two studies reported on the association of vitamin B12 levels on cognitive outcomes for those treated with metformin.27 28 In one, the apparent adverse cognitive performance associated with metformin use ceased to be statistically significant after adjustment for vitamin B12 levels.28 In the other, vitamin B12 deficiency was independently associated with lower cognitive performance in metformin users.27
Sulfonylureas
Four studies investigated the effects of sulfonylurea treatment with two included in the meta-analysis.29 30 The pooled RR for incident dementia for patients treated with sulfonylurea was 0.96 (95% CI 0.69 to 1.34). Two studies could not be incorporated because the comparisons were not against placebo. In one, the use of a sulfonylurea was associated with increased risk of dementia compared with metformin (HR 1.24; 95% CI 1.10 to 1.40).25 The other found a trend toward reduced risk of dementia with sulfonylurea use (HR 0.75; 95% CI 0.50 to 1.13).24
Thiazolidinediones
Seven studies evaluated the use of thiazolidinediones (TZDs) with two included in the meta-analysis that allowed six comparisons based on differing dose and duration of use.20 36 Pooled results showed a 29% decreased risk of dementia with TZDs compared with placebo (RR 0.71; 95% CI 0.55 to 0.93). One study reported that, compared with non-exposed individuals, pioglitazone users had a 33% reduced risk of dementia (HR 0.77; 95% CI 0.62 to 0.96) with the greatest risk reduction at both the highest cumulative doses (HR 0.50; 95% CI 0.34 to 0.75) and longest durations of use (HR 0.53; 95% CI 0.36 to 0.77; for >536 days vs no use).36
Dipeptidyl peptidase-4 inhibitor
Two studies assessed the association of dipeptidyl peptidase-4 (DPP-4) inhibitors on cognitive function,37 38 but neither could be included in our meta-analysis. In patients with DM, with and without dementia at baseline, MMSE scores improved with sitagliptin.37 Among nursing home residents with DM, there were fewer hospitalizations for cognitive issues among residents prescribed DPP-4 inhibitors compared with sulfonylureas.38
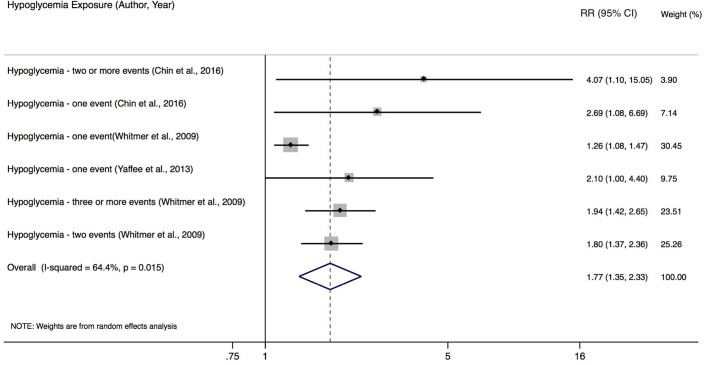
Hypoglycemia
Seven studies investigated the association between one or more hypoglycemic episodes and cognitive function. Three of these reported on severe hypoglycemia (ie, requiring immediate medical assistance) and were included in the meta-analysis.39–41 The risk of developing dementia was nearly double with the occurrence of severe hypoglycemia (RR 1.77; 95% CI 1.35 to 2.33) (figure 3). There was an increased risk of harm seen across all studies. Four studies were not included in meta-analysis because RRs could not be calculated.42–45 In general, hypoglycemia was associated with cognitive decline.
Figure 3.
Relative risk of developing dementia based on to the occurrence of one or more hypoglycemic events. RR, risk ratio.
Discussion
While the association between DM and dementia is well established, the potential impact of DM treatment on the risk of incident dementia or progression of pre-existing cognitive impairment is less clear. In our systematic review and meta-analysis, we found differential effects across drug classes with a signal of harm associated with insulin therapy, but a potential protective effect with TZD exposure. Potentially related, the occurrence of severe hypoglycemia (a risk of insulin and sulfonylurea therapy, but not metformin or TZDs) increased the likelihood of incident dementia nearly twofold. Online supplementary table 4 46 47 provides proposed mechanisms to explain potential relationships between the treatment of DM and dementia that might explain differential effects between therapies.46 47
The risk of cognitive impairment increases with the duration and severity of DM.17 48 These factors, which should be considered when assessing the association between DM treatment and cognitive outcomes, were not accounted for in many studies. Whether insulin directly increases the risk of incident dementia and other adverse cognitive outcomes remains uncertain. The apparent association may be driven, at least in part, by DM duration and severity, though severe hypoglycemia related to insulin treatment is another plausible mechanism.
The association between metformin and dementia is complex. In one clinical trial, the use of metformin in newly treated patients with DM reduced the risk of MCI.49 Our systematic review found highly variable results across studies. The meta-analysis did not demonstrate any significant association between metformin use and adverse cognitive outcomes, though significant between-study heterogeneity was seen. Vitamin B12 deficiency, which is associated with metformin use,49 may be a potential factor that may adversely affect cognition. Other potential reasons for study heterogeneity may relate to unreported differences in DM severity, co-intervention, and comorbidity.
Two studies reported a protective association with pioglitazone on the risk of dementia with this benefit directly related to higher dosage and longer duration of exposure.36 This observation warrants further study. There are two ongoing phase III trials examining the potential impact of pioglitazone on the course of AD.50
A complex, possibly bidirectional relationship exists between severe hypoglycemia and cognitive deficits. Severe hypoglycemia may cause neurological impairment, and impaired cognition may increase the risk of hypoglycemia. Studies have consistently shown that diabetic patients with cognitive dysfunction are more likely to experience hypoglycemia.51–54 Accordingly, clinical practice guidelines from the American College of Physicians recommend that in diabetic patients with dementia, treatment should focus on avoidance of symptomatic hyperglycemia, rather than achieving a strict A1c target in order to mitigate the risk of hypoglycemia.55
Future research directions
Pilot studies have reported benefit with intranasal insulin in individuals with AD.56 Further clinical trials are ongoing.57 In contrast to subcutaneous injections, intranasal insulin crosses the blood–brain barrier and poses a lower risk of hypoglycemia.58 A systematic review reported improvements in verbal memory and functional status with intranasal insulin, compared with placebo, but no apparent effect on other cognitive domains.58
Limitations
Our findings should be interpreted in the context of the study design. The major limitation of our study was the inability to account for the duration or severity of diabetes because of inconsistent reporting in the primary studies. The majority of included studies were observational in nature. Inherent to all observational studies, residual confounding cannot be excluded. Among the four RCTs that were included, study quality was generally poor, which may have impacted our estimates. Furthermore, we quantified a large amount of heterogeneity, likely arising from true clinical differences between studies. Statistically combining heterogeneous data may be problematic. Although we were able to account for some of the observed heterogeneity in our stratified and meta-regression analyses, the results from these subgroups should be interpreted to be hypothesis-generating. Although heterogeneity was also seen in our estimates of harm associated with severe hypoglycemia, the direction of association was consistently reported in every study, thus strengthening the likelihood of a true association.
Conclusions
The various antidiabetic therapies may have differential effects on cognitive outcomes, potentially mediated by risk of severe hypoglycemia. Future studies should consider DM duration and severity, and frequency and severity of hypoglycemia in assessing the complex association between DM and cognitive impairment.
Acknowledgments
We wish to acknowledge Dr Diane Lorenzetti and Zahra Premji who assisted us in the development of the search strategy. Guarantor: JMM.
Footnotes
Contributors: JMM: literature search, study design, data collection, data analysis and interpretation, and writing. BSM: literature search, study design, data collection, data analysis and interpretation, and writing. DBH: study design, data analysis and interpretation, and writing. AAL: study design, data analysis and interpretation, and writing.
Funding: The Brenda Strafford Foundation Chair in Geriatric Medicine has provided funding for Open Access Publication. JMM has received an academic scholarship for tuition for Graduate Studies. BSM has received an academic scholarship from the Government of Alberta. AAL is a recipient of the Hypertension Canada New Investigator Award.
Disclaimer: The funding sources had no role in the study design or decision to submit the manuscript for publication.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Katon W, Pedersen HS, Ribe AR, et al. . Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA Psychiatry 2015;72:612–9. 10.1001/jamapsychiatry.2015.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Umegaki H, Kawamura T, Umemura T, et al. . Factors associated with cognitive decline in older adults with type 2 diabetes mellitus during a 6-year observation. Geriatr Gerontol Int 2015;15:302–10. 10.1111/ggi.12273 [DOI] [PubMed] [Google Scholar]
- 3. Zhang J, Chen C, Hua S, et al. . An updated meta-analysis of cohort studies: diabetes and risk of Alzheimer's disease. Diabetes Res Clin Pract 2017;124:41–7. 10.1016/j.diabres.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 4. Cheng G, Huang C, Deng H, et al. . Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484–91. 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- 5. Gudala K, Bansal D, Schifano F, et al. . Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig 2013;4:640–50. 10.1111/jdi.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Secnik J, Cermakova P, Fereshtehnejad SM, et al. . Diabetes in a large dementia cohort: clinical characteristics and treatment from the Swedish dementia registry. Diabetes Care 2017;40:1159–66. 10.2337/dc16-2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bordier L, Doucet J, Boudet J, et al. . Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes Metab 2014;40:331–7. 10.1016/j.diabet.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 8. Moher D, Shamseer L, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells G, Shea B, O'Connell D, 2018. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analysis. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 10. Higgins JP, Altman DG, Gøtzsche PC, et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen G, Hemmelgarn B, Alhaider S, et al. . Meta-analysis of adverse cardiovascular outcomes associated with antecedent hypertension after myocardial infarction. Am J Cardiol 2009;104:141–7. 10.1016/j.amjcard.2009.02.048 [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parikh NM, Morgan RO, Kunik ME, et al. . Risk factors for dementia in patients over 65 with diabetes. Int J Geriatr Psychiatry 2011;26:749–57. 10.1002/gps.2604 [DOI] [PubMed] [Google Scholar]
- 14. Ma F, Wu T, Miao R, et al. . Conversion of mild cognitive impairment to dementia among subjects with diabetes: a population-based study of incidence and risk factors with five years of follow-up. J Alzheimers Dis 2015;43:1441–9. 10.3233/JAD-141566 [DOI] [PubMed] [Google Scholar]
- 15. Ott A, Stolk RP, van Harskamp F, et al. . Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology 1999;53:1937–42. 10.1212/WNL.53.9.1937 [DOI] [PubMed] [Google Scholar]
- 16. Ott A, Stolk RP, Hofman A, et al. . Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia 1996;39:1392–7. 10.1007/s001250050588 [DOI] [PubMed] [Google Scholar]
- 17. Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70–81 years. BMJ 2004;328:548 10.1136/bmj.37977.495729.EE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fei M, Yan Ping Z, Ru Juan M, et al. . Risk factors for dementia with type 2 diabetes mellitus among elderly people in China. Age Ageing 2013;42:398–400. 10.1093/ageing/afs188 [DOI] [PubMed] [Google Scholar]
- 19. Murata Y, Kadoya Y, Yamada S, et al. . Cognitive impairment in elderly patients with type 2 diabetes mellitus: prevalence and related clinical factors. Diabetol Int 2017;8:193–8. 10.1007/s13340-016-0292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heneka MT, Fink A, Doblhammer G. Effect of pioglitazone medication on the incidence of dementia. Ann Neurol 2015;78:284–94. 10.1002/ana.24439 [DOI] [PubMed] [Google Scholar]
- 21. Kuo SC, Lai SW, Hung HC, et al. . Association between comorbidities and dementia in diabetes mellitus patients: population-based retrospective cohort study. J Diabetes Complications 2015;29:1071–6. 10.1016/j.jdiacomp.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 22. Plastino M, Fava A, Pirritano D, et al. . Effects of insulinic therapy on cognitive impairment in patients with Alzheimer disease and diabetes mellitus type-2. J Neurol Sci 2010;288:112–6. 10.1016/j.jns.2009.09.022 [DOI] [PubMed] [Google Scholar]
- 23. Bruce DG, Davis WA, Starkstein SE, et al. . Mid-life predictors of cognitive impairment and dementia in type 2 diabetes mellitus: the Fremantle Diabetes Study. J Alzheimers Dis 2014;42(Suppl 3):S63–S70. 10.3233/JAD-132654 [DOI] [PubMed] [Google Scholar]
- 24. Huang CC, Chung CM, Leu HB, et al. . Diabetes mellitus and the risk of Alzheimer's disease: a nationwide population-based study. PLoS One 2014;9:e87095 10.1371/journal.pone.0087095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitmer R, Quesenberry Jr C, Allison J. Anti-hyperglycemic therapy and risk of dementia: a new user cohort study. Alzheimer's and dementia 2013;1:P136. [Google Scholar]
- 26. Lane E, Lui D, Lu Z, et al. . The differential impact of diabetes medications on cognition. Alzheimer's and dementia 2013;1:P206. [Google Scholar]
- 27. Biemans E, Hart HE, Rutten GE, et al. . Cobalamin status and its relation with depression, cognition and neuropathy in patients with type 2 diabetes mellitus using metformin. Acta Diabetol 2015;52:383–93. 10.1007/s00592-014-0661-4 [DOI] [PubMed] [Google Scholar]
- 28. Moore EM, Mander AG, Ames D, et al. . Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 2013;36:2981–7. 10.2337/dc13-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng C, Lin CH, Tsai YW, et al. . Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci 2014;69:1299–305. 10.1093/gerona/glu073 [DOI] [PubMed] [Google Scholar]
- 30. Hsu CC, Wahlqvist ML, Lee MS, et al. . Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 2011;24:485–93. 10.3233/JAD-2011-101524 [DOI] [PubMed] [Google Scholar]
- 31. Kuan YC, Huang KW, Lin CL, et al. . Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuropsychopharmacol Biol Psychiatry 2017;79:77–83. 10.1016/j.pnpbp.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 32. Orkaby AR, Cho K, Cormack J, et al. . Metformin vs sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology 2017;89:1877–85. 10.1212/WNL.0000000000004586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsiao HW, Yang YS, YL L. Risk for Alzheimer's disease in type 2 diabetic patients treated with metformin. Diabetes 2014;63:A372. [Google Scholar]
- 34. Naharci MI, Cintosun U, Ozturk A. Association of metformin therapy with the risk of dementia in older adults with type 2 diabetes mellitus. European Geriatric Medicine 2016;7:S62–S3. [Google Scholar]
- 35. Ng TP, Feng L, Yap KB, et al. . Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 2014;41:61–8. 10.3233/JAD-131901 [DOI] [PubMed] [Google Scholar]
- 36. Chou PS, Ho BL, Yang YH. Effects of pioglitazone on the incidence of dementia in patients with diabetes. J Diabetes Complications 2017;31:1053–7. 10.1016/j.jdiacomp.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 37. Isik AT, Soysal P, Yay A, et al. . The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer's disease. Diabetes Res Clin Pract 2017;123:192–8. 10.1016/j.diabres.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 38. Zullo AR, Gutman R, Mor V. The effect of dipeptidyl peptidase-4 inhibitors versus sulfonylureas on mental status, cognition, and physical functioning in older nursing home residents. J Am Geriatr Soc 2017;65:S220–S1. [Google Scholar]
- 39. Yaffe K, Falvey CM, Hamilton N, et al. . Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013;173:1300–6. 10.1001/jamainternmed.2013.6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whitmer RA, Karter AJ, Yaffe K, et al. . Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–72. 10.1001/jama.2009.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chin SO, Rhee SY, Chon S, et al. . Hypoglycemia is associated with dementia in elderly patients with type 2 diabetes mellitus: an analysis based on the Korea National Diabetes Program Cohort. Diabetes Res Clin Pract 2016;122:54–61. 10.1016/j.diabres.2016.09.027 [DOI] [PubMed] [Google Scholar]
- 42. KH H, Jeon JY, Kim HJ. Severe hypoglycemia and risk of dementia in person with diabetes mellitus. Journal of Diabetes Investigation 2017;8:35. [Google Scholar]
- 43. Mehta HB, Mehta V, Goodwin JS. Association of hypoglycemia with subsequent dementia in older patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2017;72:1110–6. 10.1093/gerona/glw217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruce DG, Davis WA, Nelson M, et al. . Severe hypoglycaemia does not explain the relationship between long duration insulin therapy and late-life cognitive impairent in type 2 diabetes: the Fremantle Diabetes Study. Alzheimer's & Dementia 2014;10:P295 10.1016/j.jalz.2014.04.490 [DOI] [Google Scholar]
- 45. Feinkohl I, Aung PP, Keller M, et al. . Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2014;37:507–15. 10.2337/dc13-1384 [DOI] [PubMed] [Google Scholar]
- 46. Luchsinger JA. Type 2 diabetes and cognitive impairment: linking mechanisms. J Alzheimers Dis 2012;30(Suppl 2):S185–S198. 10.3233/JAD-2012-111433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neil WP, Hemmen TM. Neurologic manifestations of hypoglycemia. Diabetes—Damages and Treatments: InTech, 2011. [Google Scholar]
- 48. Luchsinger JA, Diabetes LJ. Diabetes, related conditions, and dementia. J Neurol Sci 2010;299:35–8. 10.1016/j.jns.2010.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luchsinger JA, Perez T, Chang H, et al. . Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 2016;51:501–14. 10.3233/JAD-150493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galimberti D, Scarpini E. Pioglitazone for the treatment of Alzheimer's disease. Expert Opin Investig Drugs 2017;26:97–101. 10.1080/13543784.2017.1265504 [DOI] [PubMed] [Google Scholar]
- 51. de Galan BE, Zoungas S, Chalmers J, et al. . Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: preterax and diamicron modified release controlled evaluation (ADVANCE) trial. Diabetologia 2009;52:2328–36. 10.1007/s00125-009-1484-7 [DOI] [PubMed] [Google Scholar]
- 52. Feil DG, Rajan M, Soroka O, et al. . Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc 2011;59:2263–72. 10.1111/j.1532-5415.2011.03726.x [DOI] [PubMed] [Google Scholar]
- 53. Bruce DG, Davis WA, Casey GP, et al. . Severe hypoglycaemia and cognitive impairment in older patients with diabetes: the Fremantle Diabetes Study. Diabetologia 2009;52:1808–15. 10.1007/s00125-009-1437-1 [DOI] [PubMed] [Google Scholar]
- 54. Punthakee Z, Miller ME, Launer LJ, et al. . Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787–93. 10.2337/dc11-1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qaseem A, Wilt TJ, Kansagara D, et al. . Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018;168:569 10.7326/M17-0939 [DOI] [PubMed] [Google Scholar]
- 56. Craft S, Claxton A, Baker LD, et al. . Effects of regular and long-acting insulin on cognition and Alzheimer's disease biomarkers: a pilot clinical trial. J Alzheimers Dis 2017;57:1325–34. 10.3233/JAD-161256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Craft S, Aisen P, National Institute on Aging . Study of Nasal Insulin to Fight Forgetfulness (SNIFF). United States: US: Department of Health and Human Services, 2013. [Google Scholar]
- 58. Avgerinos KI, Kalaitzidis G, Malli A, et al. . Intranasal insulin in Alzheimer's dementia or mild cognitive impairment: a systematic review. J Neurol 2018;265:1497–510. 10.1007/s00415-018-8768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2018-000563supp001.docx (1.8MB, docx)