Table 1.
Diastereoselectivity of iodine-promoted carbamate annulations.
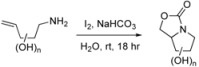 | ||||
|---|---|---|---|---|
| Entry | Alkenylamine | Carbamate | Diastereoselectivity | Yielda |
| 1 | 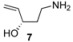 |
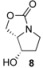 |
> 20:1 | 95% |
| 2b | 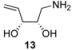 |
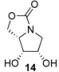 |
> 20:1 | 93% |
| 3b | 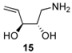 |
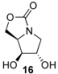 |
> 20:1 | 99% |
a Isolated yield. b See reference [11].
Diastereoselectivity of iodine-promoted carbamate annulations.
 | ||||
|---|---|---|---|---|
| Entry | Alkenylamine | Carbamate | Diastereoselectivity | Yielda |
| 1 |  |
 |
> 20:1 | 95% |
| 2b |  |
 |
> 20:1 | 93% |
| 3b |  |
 |
> 20:1 | 99% |
a Isolated yield. b See reference [11].