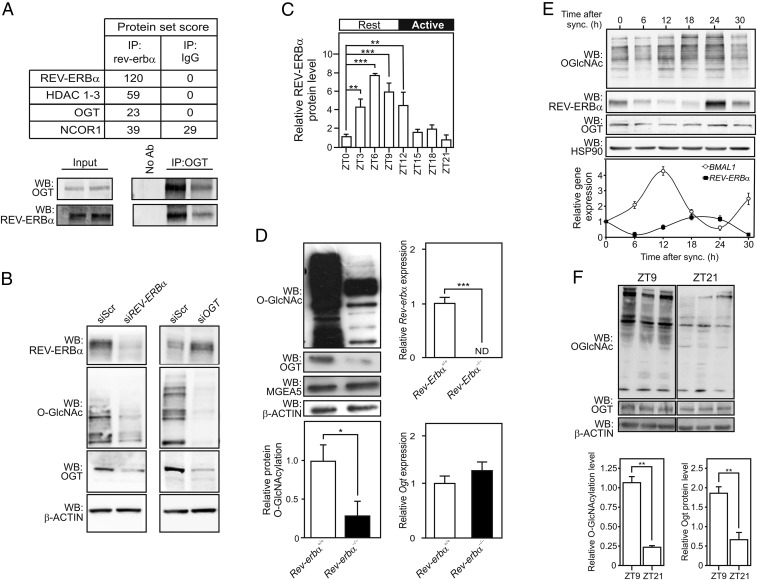
Fig. 1.
REV-ERBα interacts with OGT and controls O-GlcNAcylation. (A) Most-relevant proteins identified by RIME in HepG2 cells extract (Upper). Validation of the REV-ERBα/OGT interaction by anti-OGT coimmunoprecipitation (IP) of HepG2 whole cellular extract followed by anti-OGT and anti-REV-ERBα WB (n = 2; Lower). (B) O-GlcNAcylation levels in cellular extracts from HepG2 cells depleted from REV-ERBα or OGT mRNAs. (C) Time-dependent REV-ERBα protein expression level in mouse livers. (D) O-GlcNAcylation, OGT, and OGA proteins levels in protein extracts from WT or Rev-erbα−/−mouse livers at ZT6. Rev-erbα and Ogt mRNA levels were assessed by RT-qPCR. ND, not detectable. (E) O-GlcNAcylation, REV-ERBα, and OGT protein levels (Upper) and REV-ERBα and BMAL1 relative gene expression (Lower) in synchronized U2OS cells. (F) O-GlcNAcylation and OGT protein levels in mouse livers at ZT9 and ZT21. Results are expressed as mean ± SEM, and values were compared by a two-way ANOVA followed by a Bonferroni post hoc test (C) or a t test (D and F). *P < 0.05, **P < 0.01, ***P < 0.001.