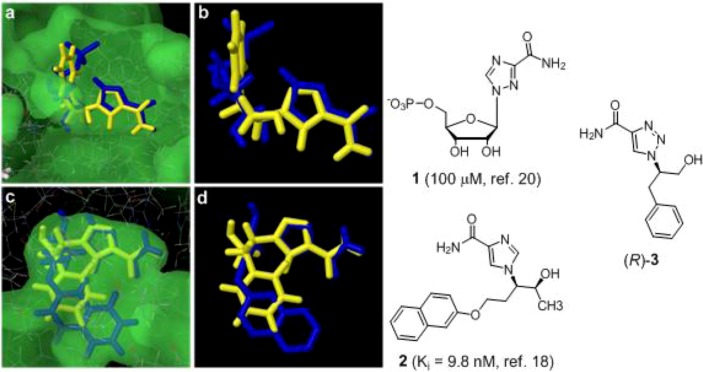
Figure 1.
(a) Docked binding mode of (R)-3 (yellow) compared to X-ray structure (1R6A) of ribavirin-5’-triphosphate (blue) 1 in the active site of the NS5MTaseDV in the dengue virus; (b) The docking shows that the NH2-groups of compound (R)-3 and ribavirin-5’-monophosphate are almost superimposed and makes the same important interaction with the carbonyl functions of Leu17 and Leu20 in the protein; (c) Docked binding mode of (R)-3 (yellow) compared to x-ray structure (1v7a) of inhibitor 2 in the active site of the ADA enzyme; (d) The imidazole part of compound (R)-3 is almost superimposed over compound 2 and the phenyl ring points in (R)-3 in the same direction as the naphtyl ring in 2.