Table 2.
Synthesis of 1,4-disubstituted-1,2,3-triazoles.
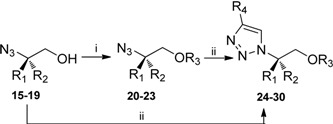
| Compound | R1 | R2 | R3 | R4 | Yield (%) |
|---|---|---|---|---|---|
| 20 | H | CH2Ph | CH2Ph | - | 87 |
| 21 | CH2Ph | H | CH2Ph | - | 91 |
| 22 | H | CH2CH2Ph | CH2Ph | - | 92 |
| 23 | H | CH2OCH2Ph | Si(CH3)2C(CH3)3 | - | 67 |
| 24 | H | CH2Ph | H | CO2CH3 | 71 |
| 25 | H | CH(CH3)2 | H | CO2CH3 | 74 |
| 26 | H | CH2Ph | H | Ph | 97 |
| 27 | H | CH2Ph | CH2Ph | CO2CH3 | 83 |
| 28 | CH2Ph | H | CH2Ph | CO2CH3 | 90 |
| 29 | H | CH2CH2Ph | CH2Ph | CO2CH3 | 82 |
(i) BnCl, NaH, TBAI, THF, 15 h, RT; or TBDMSiCl, imidazole, DMF, 15 h, RT; (ii) sodium ascorbate, CuSO4 in H2O:t-BuOH (1:1 v/v), 15h, RT.
