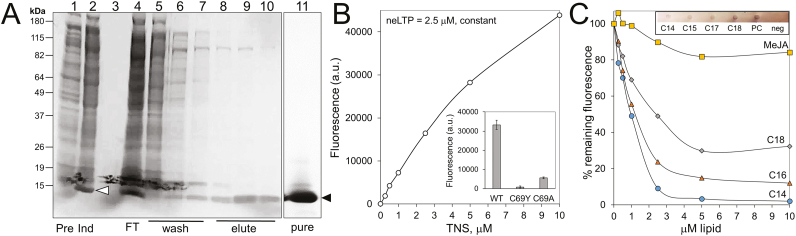
Fig. 5.
Heterologously expressed BrLTP2.1 has lipid binding activity. (A) Heterologously expressed and purified BrLTP2.1 from E. coli. Lane 1: pre-induction E. coli lysate; lane 2: 4 h post-induction lysate; lane 3: cell media; lane 4: flow-through from Co2+ affinity column; lanes 5–7: column washes; lanes 8–10: elutions with 300 mM imidazole; lane 11: pure protein after concentration and desalting. (B) TNS, a lipophilic fluorophore binds to BrLTP2.1. TNS concentration ranged from 0 to 10 μM, while BrLTP2.1 concentration was held constant at 2.5 μM, with excitation at 320 nm and emission recorded at 437 nm. Inset: TNS-dependent fluorescence in wild-type and two mutant versions of BrLTP2.1 (protein and TNS both at 2.5 μM). (C) Lipids present in Brassica nectars competitively displaced TNS from BrLTP2.1. TNS and BrLTP2.1 concentration were each held constant at 2.5 μM, while myristic acid (C14), palmitic acid (C16), stearic acid (C18), and methyl jasmonate (MeJA) ranged from 0 to 10 μM. Inset: Dot blot analysis of BrLTP2.1 binding to myristic (C14), pentadecanoic (C15), heptadecanoic (C17), and stearic (C18) acid, as well as phosphatidylcholine (PC). A 2:1:0.8 solution of methanol:chloroform:water, the solvent for all lipids, was used as a negative control (neg). BrLTP2.1 binding was detected with anti-His antibodies.