FIGURE 2.
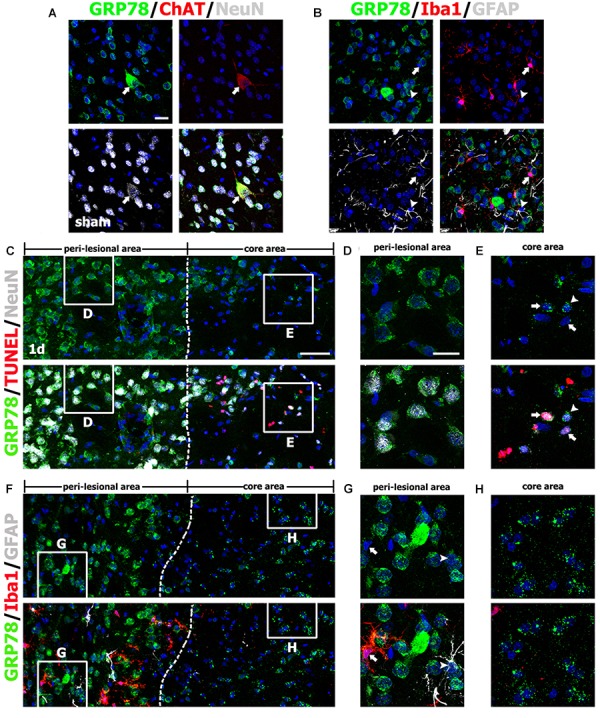
Characterization of glucose-regulated protein (GRP78)-positive cells in the striata of saline-treated control and 3-nitropropionic acid-injected rats on day 1. (A) Triple-labeling of GRP78, neuronal nuclear protein (NeuN), and choline acetyltransferase (ChAT), a specific marker for cholinergic neurons, in control striatum, showing that GRP78 expression is localized in almost all NeuN-positive neurons and also in some cholinergic interneurons (arrows). (B) Triple-labeling of GRP78, ionized calcium-binding adaptor molecule 1 (Iba1), and glial fibrillary acidic protein (GFAP) in the striatum of a control rat, showing that neither microglia (arrows) nor astrocytes (arrowheads) show specific immunoreactivity for GRP78. (C–E) Lower- (C) and higher- (D,E) magnification views of sections triple-labeled for GRP78, NeuN, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) 1 day after lesion induction. The boxed areas of the peri-lesional area (left side of the broken line) and the lesion core in C are enlarged in D and E, respectively. Notably, GRP78 expression is very weak to negligible in nearly all TUNEL-positive (arrows in E) and TUNEL-negative (arrowheads in E) neurons in the lesion core, while neurons in the peri-lesional area show prominent GRP78 immunoreactivity. (F–H) Lower- (F) and higher- (G,H) magnification views of section triple-labeled for GRP78, Iba1, and GFAP 1 day after lesion induction. The boxed areas of the peri-lesional area (left side of the broken line) and the lesion core in F are enlarged in G and H, respectively. Note that astrocytes (arrowheads in G) and microglia (arrows in G) in the peri-lesional area show very weak immunoreactivity for GRP78. Cell nuclei are stained with 4′,6-diamidino-2-phenylindole. Scale bars represent 50 μm for C, F; and 20 μm for A, B, D, E, G, H.
