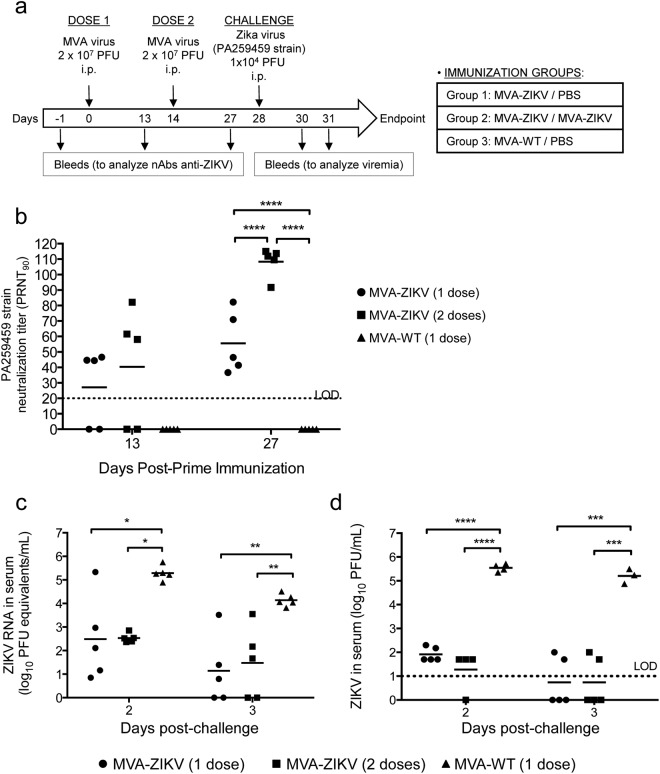
Figure 6.
Efficacy of MVA-ZIKV in immunocompromised susceptible IFNAR−/− mice. (a) Inoculation scheme. Groups of IFNAR−/− mice (n = 10 mice/group) were immunized with 2 × 107 PFUs of MVA-WT (one dose, at day 0) or MVA-ZIKV (one or two doses, at days 0 and 14, respectively) by the i.p. route. Twenty eight days after the first immunization, mice were challenged with 104 PFUs of ZIKV (PA259459, strain Panama) via the i.p. route (see Methods). (b) ZIKV-neutralizing antibody titers (PRNT90) detected at 13 and 27 days post-prime immunization in sera (n = 5) of animals immunized with one dose of MVA-WT or one or two doses of MVA-ZIKV. Titers of neutralizing antibodies against ZIKV PA259459 strain were determined by a PRNT assay and are expressed as the reciprocal of the serum dilution that inhibited plaque formation by 90% (PRNT90), relative to samples incubated with negative control sera (from day 1 pre-prime). Dashed line indicates the limit of detection (LOD) of the neutralization assay (1/20 dilution). The statistically significant difference between the groups is indicated (****P < 0.0001). (c) ZIKV RNA viremia after challenge. Blood samples were collected at days 2 (n = 5) or 3 (n = 5) post-challenge, and ZIKV RNA viremia was analyzed by quantitative real-time PCR (PFU equivalents/ml). Graph shows mean with each point representing an individual mouse. P values indicate significantly higher responses between the different groups (*P < 0.05; **P < 0.005). (d) ZIKV infectious virus after challenge. Blood samples were collected at days 2 (n = 5) or 3 (n = 5) post-challenge, and ZIKV infectious virus was analyzed by a plaque assay (PFUs/ml). Graph shows mean with each point representing an individual mouse. P values indicate significantly higher responses between the different groups (***P < 0.001, ****P < 0.0001).