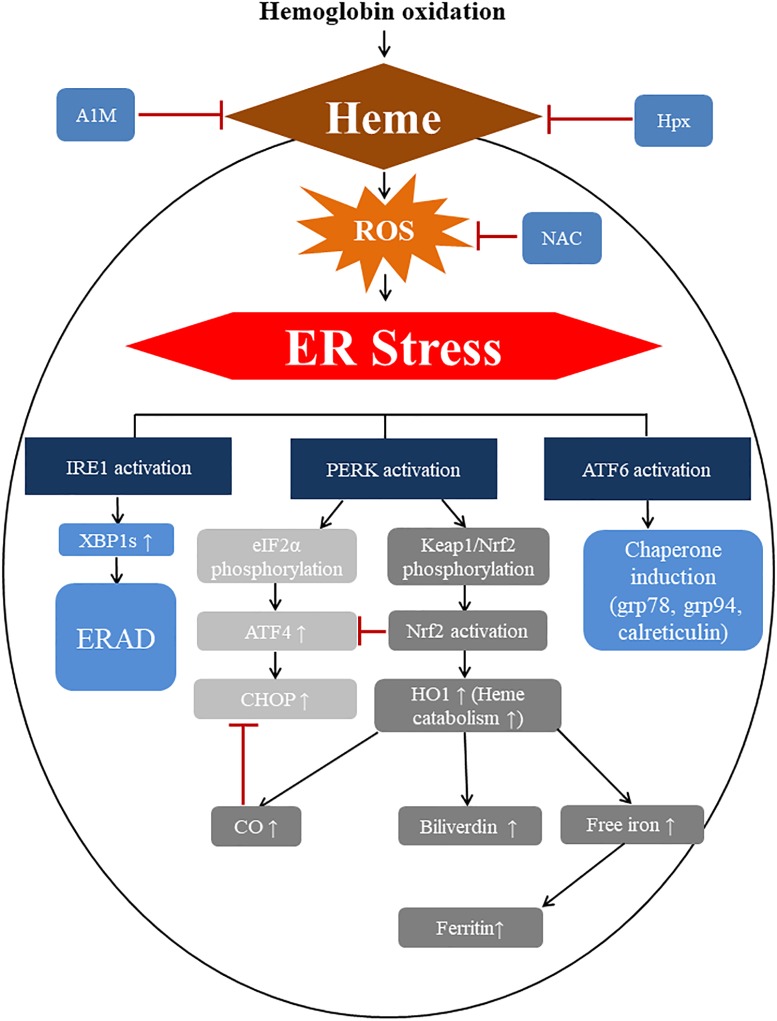
FIGURE 13.
Mechanism of heme induced ER stress with possible therapeutic interventions. Heme released from oxidized hemoglobin enters into cells across the plasma membrane (by unknown mechanisms) possibly via passive diffusion or transporters (remains to be defined) and induces ROS intracellularly with subsequent protein oxidative damage that leads PERK activation, phosphorylation of eIF2α with subsequent ATF4 as well as CHOP induction and nuclear translocation. Oxidation of Keap1 releases Nrf2, which PERK is known to phosphorylate Keap1/Nrf2 followed by nuclear translocation of Nrf2, which interacts with various targets and possibly influences CHOP expression directly as well as indirectly. For example, Nrf2 might directly inhibit ATF4 binding to CHOP promoter thereby inhibiting ATF4-mediated CHOP expression. Nrf2 also induces HO1, one of the key heme responsive genes. HO1 decreases the heme burden of cells and releases heme catabolites such as carbon-monoxide (CO), biliverdin (which is converted to the antioxidant bilirubin by biliverdin reductase in the cytosol), and iron (Fe2+). CO might inhibit CHOP expression, which suggests that Nrf2 may negatively influence CHOP in an indirect, HO1-mediated way as well. Activation of XBP1s through IRE1 in response to heme possibly induces ER-associated protein degradation (ERAD), which decreases misfolded protein loads and reduces unfolded protein burden of ER. Heme-induced activation of ATF6 also facilitates chaperone expression like Grp78 increasing the folding capacity of ER. Thus, heme potentially activates both pro-apoptotic and pro-survival ER responses, however, our data support that the pro-apoptotic response is only transitory, while pro-survival ones (ATF6, Grp78) are permanently activated. The observed marked induction of heme responsive genes as well as ER stress responsive genes suggest that these pathways interact with each other to cope with heme-induced stress. Finally, capturing heme in the extracellular space by the heme binding proteins alpha-1-microglobulin and hemopexin or decreasing reactive oxygen species level by NAC attenuate heme-induced ER stress.