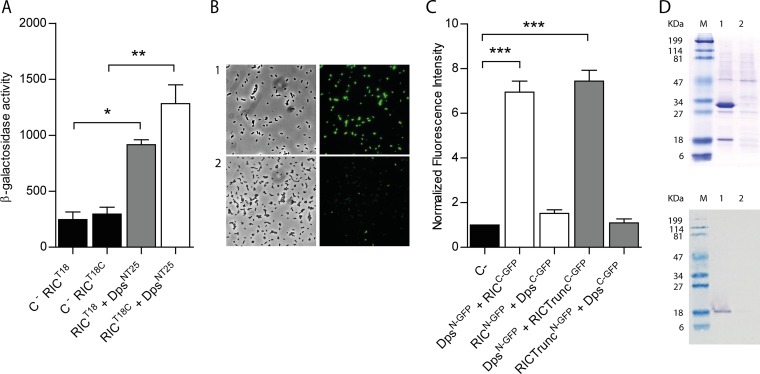
FIG 2.
E. coli RIC interacts with Dps. (A) Bacterial two-hybrid assay. The interaction of the RIC protein, linked to the C terminal (white bar) or N terminal (gray bar) of the T18-Cya domain and expressed from pUT18 or pUT18C, respectively, was evaluated in E. coli DHM1 cotransformed with pKTN25 containing a Dps linked to the N terminal of the T25-Cya domain. E. coli cells harboring simultaneously empty pKTN25 and pUT18/pUT18C vectors expressing ric fusions served as negative controls (black bars). (B and C) BiFC assays. Cells were cotransformed with vectors expressing either RICC-GFP (pMRBAD-link-C-GFP-RIC) or RIC-TruncatedC-GFP (pMRBAD-link-C-GFP-RICTrunc) with DpsN-GFP (pET11a-link-N-GFP-Dps). The inverse configurations were also included. (B) Cells expressing RICC-GFP and DpsN-GFP were analyzed by light microscopy (bright field, left upper panel) and fluorescence microscopy (right upper panel). Lower panels depict images of cotransformed cells with empty vectors. Images were acquired using a 100× objective, and a fluorescein isothiocyanate (FITC) filter was used for the acquisition of the fluorescence images. (C) Fluorescence quantification was performed using MetaMorph microscopy automation and image analysis software. Fluorescence values for the negative control (empty plasmid vectors) were normalized to 1. (D) Pulldown assays. Lane 1, cells expressing pET-28a-RIC(HisTag) (RIC protein linked to a N-terminal His-tagged RIC [∼30 kDa]) and pACYCDuet-1-Dps (nonlabeled Dps [∼18 kDa]). Lane 2, cells expressing pET-28a (empty vector) and pACYCDuet-1-Dps (nonlabeled Dps [∼18 kDa]). Protein fractions were eluted from the Ni-chelating column at 100 mM imidazole and analyzed by SDS-PAGE (upper panel) and Western blotting using the anti-E. coli Dps antibody (bottom panel). Values are means ± standard errors from at least three independent cultures analyzed in duplicate. ***, P < 0.0005 (one-way analysis of variance [ANOVA] multiple-comparison test).