Mass drug administration (MDA) of sequential rounds of antimalarial drugs is being considered for use as a tool for malaria elimination. As an effective and long-acting antimalarial, dihydroartemisinin-piperaquine (DHA-PQP) appears to be suitable as a candidate for MDA.
KEYWORDS: cardiac safety, cardiotoxicity, dihydroartemisinin-piperaquine, electrocardiography, malaria
ABSTRACT
Mass drug administration (MDA) of sequential rounds of antimalarial drugs is being considered for use as a tool for malaria elimination. As an effective and long-acting antimalarial, dihydroartemisinin-piperaquine (DHA-PQP) appears to be suitable as a candidate for MDA. However, the absence of cardiac safety data following repeated administration hinders its use in the extended schedules proposed for MDA. We conducted an interventional study in Lihir Island, Papua New Guinea, using healthy individuals age 3 to 60 years who received a standard 3-day course of DHA-PQP on 3 consecutive months. Twelve-lead electrocardiography (ECG) readings were conducted predose and 4 h after the final dose of each month. The primary safety endpoint was QT interval correction (QTc using Fridericia’s correction [QTcF]) prolongation from baseline to 4 h postdosing. We compared the difference in prolongations between the third course postdose and the first course postdose. Of 84 enrolled participants, 69 (82%) participants completed all treatment courses and ECG measurements. The average increase in QTcF was 19.6 ms (standard deviation [SD], 17.8 ms) and 17.1 ms (SD, 17.1 ms) for the first-course and third-course postdosing ECGs risk difference, −2.4 (95% confidence interval [95% CI], −6.9 to 2.1; P = 0.285), respectively. We recorded a QTcF prolongation of >60 ms from baseline in 3 (4.3%) and 2 (2.9%) participants after the first course and third course (P = 1.00), respectively. No participants had QTcF intervals of >500 ms at any time point. Three consecutive monthly courses of DHA-PQP were as safe as a single course. The absence of cumulative cardiotoxicity with repeated dosing supports the use of monthly DHA-PQP as part of malaria elimination strategies.
INTRODUCTION
Malaria elimination is set to be a global health priority in coming years, and ambitious plans for scaling up from malaria control to elimination already exist in many areas of endemicity worldwide. New strategies being actively considered for interrupting malaria transmission include mass drug administration (MDA) (1). This strategy requires the treatment of entire populations with effective antimalarial drugs to reduce the human reservoir of high and low parasite density blood-stage infections, in addition to conferring posttreatment prophylaxis that prevents reinfection and relapse over a time period that significantly exceeds the life span of the anopheline vector (2). Because most individuals treated in an MDA program are asymptomatic parasite carriers or uninfected, the ideal antimalarial for MDA must (i) have a prolonged duration of effect that optimizes the period of prophylaxis against reinfection and relapse and (ii) be demonstrated to be safe when delivered in sequential repeated treatment courses in the manner proposed for MDA deployment (2).
Dihydroartemisinin-piperaquine (DHA-PQP) is a good candidate for MDA. The rapid-acting artemisinin-derivative reduces the parasite biomass of existing infections, and the long-acting PQP component exerts an especially long posttreatment prophylactic effect (3). Repeated monthly exposure to standard 3-day treatment courses of DHA-PQP over 3 consecutive months should theoretically prevent new infections for a period of at least 90 to 120 days. In a meta-analysis of 11 studies, monthly DHA-PQP for high-risk populations was associated with an 84% reduction in the incidence of malaria parasitemia (4–6).
Nevertheless, the feasibility of DHA-PQP for MDA has been questioned because PQP can cause dose-dependent prolongation of the electrocardiographic QT interval. As such, PQP is contraindicated in patients with congenital long-QT syndrome (about one in 2,500 children) or those taking other drugs that prolong the QT. Mild QT prolongation is clinically silent, but extreme prolongation can cause fatal arrhythmias, such as torsades de pointes (TdP). Prior studies have demonstrated that QTc prolongation associated with DHA-PQP is predominantly mild and not associated with clinical adverse cardiac outcomes (4, 7). However, PQP is eliminated slowly (elimination half-life, ∼23 days), and the risk of QT prolongation may be exacerbated when repeated doses are given, especially if given monthly (8). Therefore, the drug manufacturer and the European Medicines Agency (EMA) recommend that repeated treatment courses of DHA-PQP (brand name Eurartesim) in 160-mg/20-mg film-coated tablets should not be administered within 2 months of initial treatment (9). Unfortunately, this is unlikely to be optimally effective if DHA-PQP is given as part of MDA; mathematical models suggest a maximum interval of 1 month between treatment rounds in order to interrupt transmission (10).
The current study aims to assess the cardiac safety of three repeated monthly courses of DHA-PQP in Lihir Island, Papua New Guinea (PNG), a population of endemicity for malaria likely to be targeted in future MDA activities.
RESULTS
Of 110 individuals screened, 84 (76.3%) individuals met the inclusion criteria and consented to participate in the study. Eighteen individuals declined consent, and 8 individuals were ineligible (first trimester pregnancy, n = 2; uncontrolled hypertension, n = 2: asymptomatic arrhythmia, n = 2; clinical heart failure, n = 1; and congenital heart disease, n = 1). Of the 84 participants who were initially enrolled, 69 (82.2%) participants completed all treatment courses and follow-up appointments (per-protocol population) and were included in subsequent analysis.
The mean age of participants in the per-protocol population was 27.4 years (standard deviation [SD], 12.6 years); 7 (10%) participants were children (age 3 to 15 years), and 34 (49%) participants were female. One female participant was pregnant (confirmed second trimester by dates and clinical examination). At baseline, the mean QT interval with Fridericia’s correction (QTcF) was 397.3 ms (SD, 17.2 ms). Sixty-five (94%) participants had a normal baseline ECG, and 4 (6%) participants had minor abnormalities of no clinical relevance (i.e., 3 with nonpathological T-wave inversion and 1 with poor R-wave progression). A comparison of baseline demographic, clinical, and electrocardiographic characteristics of participants who completed follow-up and those who were lost to follow-up were not significantly different (Table 1).
TABLE 1.
Comparison of baseline characteristics of participants that completed follow-up and participants that were lost to follow-upa
| Characteristic | Participant status |
P valueb | ||
|---|---|---|---|---|
| Finished study | Lost to follow-up | Total | ||
| Demographic variables | ||||
| Sex | ||||
| Male | 35 (51) | 11 (73) | 46 (55) | 0.111 |
| Female | 34 (49) | 4 (27) | 38 (84) | |
| Age (mean [SD]) (yr) | 27.4 (12.6) | 29.6 (12.8) | 27.8 (12.6) | 0.546 |
| <15 | 7 (10) | 1 (7) | 8 (10) | 1.000 |
| ≥15 | 62 (90) | 14 (93) | 76 (90) | |
| Clinical variables | ||||
| Hypertension | ||||
| No | 68 (99) | 15 (100) | 83 (99) | 1.000 |
| Yes | 1 (1) | 0 (1) | 1 (1) | |
| Diabetes | ||||
| No | 69 (100) | 15 (100) | 84 (100) | |
| Yes | 0 (0) | 0 (0) | 0 (0) | |
| Chronic treatment | ||||
| No | 68 (99) | 14 (93) | 82 (98) | 0.327 |
| Yes, not related to risk of QT enlargement | 1 (1) | 1 (7) | 2 (2) | |
| Temp (mean [SD]) (°C) | 35.5 (0.9) | 35.2 (1.1) | 35.4 (0.9) | 0.238 |
| Cardiac auscultation | ||||
| No alterations | 64 (93) | 15 (100) | 79 (94) | 0.580 |
| Presence of murmur | 5 (7) | 0 (0) | 5 (6) | |
| Splenomegaly | ||||
| No | 64 (93) | 15 (100) | 79 (94) | 0.580 |
| Yes | 5 (7) | 0 (0) | 5 (6) | |
| Blood tests | ||||
| Malaria test (blood slide) | ||||
| Negative | 63 (91) | 15 (100) | 78 (93) | 1.000 |
| Positive Plasmodium falciparum | 4 (6) | 0 (0) | 4 (5) | |
| Positive Plasmodium vivax | 1 (1) | 0 (0) | 1 (1) | |
| Positive Plasmodium malariae | 1 (1) | 0 (0) | 1 (1) | |
| Concn (mean [SD]) | ||||
| Glucose (mmol/liter) | 4.8 (3.7) | 5.4 (1.4) | 4.9 (3.4) | 0.564 |
| Creatinine (µmol/liter) | 70.7 (26.5) | 79.6 (26.5) | 70.7 (26.5) | 0.370 |
| Sodium (mmol/liter) | 139.9 (3.8) | 141.7 (1.4) | 140.2 (3.5) | 0.083 |
| Potassium (mmol/liter) | 4.0 (0.3) | 4.3 (0.3) | 4.1 (0.3) | 0.018 |
| Hemoglobin (g/dl) | 12.5 (2.1) | 13.1 (2.0) | 12.6 (2.1) | 0.309 |
| ECG parameters (mean [SD]) | ||||
| Heart rate (bpm) | 68.4 (12.4) | 73.8 (11.6) | 69.4 (12.3) | 0.127 |
| PR segment (ms) | 166.9 (18.7) | 165.1 (22.0) | 166.6 (19.2) | 0.749 |
| QRS segment (ms) | 90.9 (9.5) | 92.7 (9.2) | 91.2 (9.4) | 0.525 |
| QTcF segment (ms) | 397.3 (17.2) | 391.6 (18.8) | 396.3 (17.5) | 0.255 |
| Conclusion | ||||
| ECG normal | 65 (94) | 12 (80) | 77 (92) | 0.104 |
| Minor nonpathological abnormalities | 4 (6) | 3 (20) | 7 (8) | |
Results are described as number (%), unless otherwise specified.
P value corresponds to the results of the paired t test comparing both groups. Statistically significant results are reflected in bold.
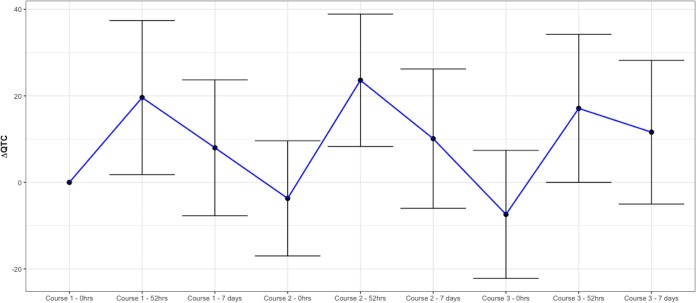
The mean (SD) ΔQTcF (from 0 h to 52 h) was 19.6 ms (17.8 ms) in course 1, 23.6 ms (15.3 ms) in course 2 (difference, 4.0 ms [95% confidence interval {95% CI}, 0.1 to 8.0 ms]; P = 0.043), and 17.1 ms (17.1 ms) in course 3 (difference, −2.4 [95% CI, −6.9 to 2.1] P = 0.285; Table 2 and Fig. 1 and 2).
TABLE 2.
Electrocardiographic measurements of QTcF and ΔQTcF at 52 h postdose during the second and third monthly course with dihydroartemisinin-piperaquine compared with the first montha
| ECG parameter |
0 hcourse1
(mean ± SD) (ms) |
52 hcourse1
(mean ± SD) (ms) |
Course 2 |
Course 3 |
||||
|---|---|---|---|---|---|---|---|---|
| 52 hcourse2
(mean ± SD) (ms) |
P value | Risk difference (95% CI) |
52 hcourse3
(mean ± SD) (ms) |
P value | Risk difference (95% CI) |
|||
| QTcF | 397.3 ± 17.2 | 416.8 ± 23.4 | 421.0 ± 19.8 | 0.040 | 4.1 (0.2 to 8.1) | 414.3 ± 23.6 | 0.270 | −2.5 (−7.1 to 2.0) |
| ΔQTcF | 19.6 ± 17.8 | 23.6 ± 15.3 | 0.043 | 4.0 (0.1 to 8.0) | 17.1 ± 17.1 | 0.285 | −2.4 (−6.9 to 2.1) | |
The 52-h readings were conducted 4 h after administration of the third dose. The parameter measurements are the arithmetic mean of measurements from triplicate readings. Statistically significant results are in bold type. P values are by paired t test for comparison with measurement of 52 hcourse1.
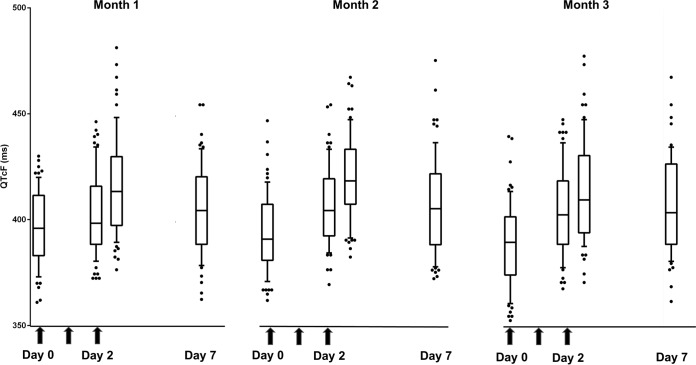
FIG 1.
Electrocardiographic QTcF measurements over the study period. Results are median and interquartile ranges (IQR) of all QTcF measurements over the 63 days of study. Arrows are the DHA-PQP doses. Day 0 corresponds to the 0-h value of each month, day 2 values correspond to 48-h (predose) and 52-h (4 h postdose) measurements, and day 7 value is the day 7 measurement of each month.
FIG 2.
Changes in the QTcF measurements (ΔQTcF) over the study period. Results are mean and standard deviation (SD) of the ΔQTcF (difference in QT corrected by Fridericia’s correction between baseline and each point of measurement) over the 63 days of study. 0 hcourse1 corresponds to day 0 predose, 52 hcourse1 corresponds to day 2 postdose, day 7course1 corresponds to day 7 postdose, 0 hcourse2 corresponds to day 28 predose, 52 hcourse2 corresponds to day 30 postdose, day 7course2 corresponds to day 35, 0 hcourse3 corresponds to day 56 predose, 52 hcourse3 corresponds to day 58 postdose, and day 7course3 corresponds to day 63.
In subgroup analyses (Table S1), the mean (SD) ΔQTcF was higher in females (25.8 ms [20.5 ms]) than in males (13.5 ms [12.2 ms]) after course 1 (P = 0.003). However, there was no significant difference in ΔQTcF values between males and females after the second and third courses (22.7 ms [15.9 ms] in males and 24.5 ms [14.9 ms] in females, P = 0.623 for the second course; and 14.3 ms [15.5 ms] in males and 20.1 ms [18.4 ms] in females, P = 0.158 for the third course). The mean QTcF segment and ΔQTcF values did not differ significantly according to age group (data not shown).
Figure 1 shows the evolution of QTcF at all time points (days 0, 2, 7, 28, 30, 35, 56, 58, and 63). Table 3 shows the resolution in ECG parameters at 7 days after the start of each DHA-PQP treatment course and prior (at 0 h) to the following course. The QTcF at 0 h for course 3 (QTcF 0 hcourse3) was shorter than QTcF 0 hcourse1 (−4.0 ms, P < 0.001), which represents a full resolution of the QTcF prolongation 28 days after the start of treatment of course 2. The QTcF and ΔQTcF parameters on 7 dayscourse2 and 7 dayscourse3 showed no differences compared to those at 7 dayscourse1 (Table S2).
TABLE 3.
Electrocardiographic measurements of QTcF and ΔQTcF at 7 days after the start of each course of DHA-PQP and before the start of the following monthly course compared with 0-h measurements on day 0 first coursea
| ECG parameter |
0 hcourse1
measurement |
7 dcourse1 |
0 hcourse2 |
7 dcourse2 |
0 hcourse3 |
7 dcourse3 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement | P value | Measurement | P value | Measurement | P value | Measurement | P value | Measurement | P value | ||
| QTcF | 397.3 ± 17.2 | 405.7 ± 20.9 | <0.001 | 393.9 ± 18.1 | 0.043 | 407.7 ± 22.7 | <0.001 | 389.9 ± 18.9 | <0.001 | 407.7 ± 22.4 | <0.001 |
| ΔQTcF | 8.0 ± 15.7 | −3.7 ± 13.3 | 10.1 ± 16.1 | −7.4 ± 14.8 | 11.0 ± 16.9 | ||||||
Results of measurements (mean ± SD, in ms) and P values for the paired t test for comparison with baseline 0-hcourse1 measurements. All P values shown are statistically significant.
No participant had cardiac-related severe adverse effects (SAEs) during the study period. Table 4 shows the recording of cardiac adverse effects of special interest (AESIs) occurring any time during the 63-day study observation period. A ΔQTcF of >60 ms was observed in 3 (4.3%), 1 (1.4%, P = 0.50), and 2 (2.9%, P = 1.00) participants after the first, second, and third courses of treatment, respectively. None of the participants had QTcF readings of more than 500 ms. QTcF readings of ≥450 to <500 ms were noted in 6 (8.9%), 5 (7.2%, P = 1.00), and 5 (7.2%, P = 1.00) participants after the first, second, and third courses of treatment, respectively. Other reported cardiac AESIs included sinus bradycardia (<40 beats per minute [bpm]) in 1 (1.4%) participant in each treatment course, and 2 (2.9%) participants with changes in T-wave morphology after the second courses of treatment. No AESIs were accompanied by clinical symptoms. Other previously defined AESIs (new bundle branch block and arrhythmia of new appearance) were not reported by any participant at any time during the study.
TABLE 4.
Adverse events of special interest recorded on 52 h of each treatment coursea
| AESI descriptionb | No. (%) |
P value for difference with course 1c |
52 hcourse3
(no. [%]) |
P value for difference with course 1c |
|
|---|---|---|---|---|---|
| 52 hcourse1 | 52 hcourse2 | ||||
| QTcF prolongation >60 ms | 3 (4.3) | 1 (1.4) | 0.50 | 2 (2.9) | 1.00 |
| QTcF ≥450 ms and <500 ms | 6 (8.7) | 5 (7.2) | 1.00 | 5 (7.2) | 1.00 |
| Sinus bradycardia (<40 bpm) | 1 (1.4) | 1 (1.4) | 1.00 | 1 (1.4) | 1.00 |
| T-wave morphologic changes | 0 (0.0) | 2 (2.9) | 0.50 | 0 (0.0) | |
Results are described in the number of cases and percentage (%) in the per-protocol study population.
AESI, adverse event of special interest. No new cases of bundle branch block or arrhythmia were described.
P value results are shown using the exact McNemar significance probability test.
Noncardiac AEs occurred in 29 (14.0%) of 207 patient visits. All reported AEs were mild and transient, with no participant requiring specialized treatment. The most commonly reported AE were abdominal pain (n = 12 [5.8%]), followed by headache (3.8%), cough (2.4%), and nausea (1.9%). All rapid diagnostic tests (RDTs) and microscopy smears collected at 0 hcourse2 and 0 hcourse3 were negative for malaria parasitemia.
Other ECG measurements (HR, ΔHR, PR, ΔPR, QRS, and ΔQRS) along the 63 days of evolution are described in Tables S3 and S4.
DISCUSSION
In this study, three monthly repeated courses of DHA-PQP resulted in our primary study endpoint, a change in the mean post-final-dose QTcF between the first and final courses, 17.7 ms; this is a magnitude generally similar to those described after a single course and lying below the U.S Food and Drug Administration’s 20-ms threshold of high level of concern (11). Our findings that QTcF prolongation did not increase in a cumulative manner with repeat courses was supported by the observation that the QTcF returned to normal prior to each subsequent course of treatment. Interestingly, the QTcF difference increased slightly (23.6 ms) after the second but not the third course. This result is intriguing and will require correlation with drug concentrations once the results related to the drug’s pharmacokinetics of the current study become available. In addition, it is reassuring that no individual had any QTcF of >500 ms and that the number of cases with an absolute increase of >60 ms were limited to 6 individuals evenly spread throughout each of the monthly courses. This therefore does not add to concerns raised by a recent large multicentric clinical trial about the possibility of increased incidence of this event after a repeated dose (7). Our results support previous findings that QT/QTc prolongation following DHA-PQP administration is consistently lower than that caused by other commonly used antimalarial drugs, such as quinine (12, 13) or chloroquine (14). Previous studies assessing the cardiotoxicity of DHA-PQP have shown minimal QTc prolongation following a single 3-day course. For example, in Cambodia, the mean (corrected by Bazett’s formula [QTcB]) prolongation in 62 individuals was 11 ms at 24 h after first dose (15), and the mean QTcF prolongation in 56 adults in Thailand was 29 ms at 52 h after the start of treatment (16). In a cohort of 1,002 malaria patients from a study conducted in four African countries, only 3 (0.3%) patients had a QTcF of >500ms after a standard 3-dose treatment (1-month course), and fewer than 10% of participants had an increase in QTcF more than 60 ms from baseline (17). A recent meta-analysis of 11 studies involving repeated exposures of DHA-PQP for seasonal malaria chemoprevention or treatment of clinical malaria found no increased incidence of AEs with repeated dosing; however, only one of these (a study of 13 pregnant women [G. Dorsey, unpublished data]) performed electrocardiographic assessments of QTc prolongation. The authors called for more studies incorporating electrocardiogram measurements (4). Recent MDA programs using DHA-PQP in large populations have shown that this drug combination is safe to use due to the minimal occurrence of serious adverse events; however, electrocardiographic assessments were not performed to monitor cardiac side effects (18–20).
Several secondary observations are worth noting, including sex-related differences in the measurements of QT-related parameters. The study showed an increase in QTcF among females at all time points that is consistent with previous studies (21, 22). Regarding AEs, less than one-fifth of patients reported mild abdominal pain, headache, cough, or nausea. All appeared to be self-limiting, and none required specific treatment or intervention. Although this trial was not designed to evaluate the efficacy of DHA-PQP, all participants remained parasite negative for the duration of the study. This lack of breakthrough infections, in an area where malaria transmission is high (5, 6, 23) and endogenous Plasmodium vivax relapses are common, reassuringly suggested that posttreatment prophylaxis was satisfactory.
This study, however, does present certain limitations. The first one includes the lack of a control group, which may hinder some of the interpretation of our results. Another limitation was the relatively high attrition rate that saw 18% of enrolled participants fail to complete the scheduled follow-up appointments, and these were therefore excluded from our per-protocol analysis. If those lost to follow-up had been prone to greater QTc prolongation, this could have been a source of bias. However, there were no statistically significant differences in baseline characteristics between participants who were lost to follow-up and those who completed follow-up. We also acknowledge an issue of generalizability of our findings regarding the way we managed food coadministation in this study. Administration of DHA-PQP with food, particularly fat, increases bioavailability, leading to increased drug concentrations and greater degree of QT prolongation (8). We carefully controlled this by advising all participants to avoid food intake for the 3 h before and after drug administration. However, while feasible in the context of a tightly controlled research study, this level of compliance may be difficult to achieve in a real MDA campaign delivered on a very large scale. Hence, it is possible that individuals not following this dietary advice could be prone to greater drug absorption, higher cumulative doses, and greater QTc prolongation than seen in our study.
Our primary endpoint was to examine changes in mean QTcF values consistent with guidelines by the U.S. FDA and other regulatory bodies. However, in terms of population risk, the way in which values are distributed across populations is perhaps more important than the measures of centrality reported here. It is those individuals who lie on the upper edge of the population distribution who are most important. For example, if an MDA intervention is deployed in 100,000 people, presuming that QTc distribution has a normal population distribution, 2,500 people will have QTc prolongations that are 2 SD above the population mean. These would be the individuals at greatest risk of a serious cardiac event. Our clinical study, like all others performed to date, did not have anywhere near the sample size required to define the population distribution accurately enough to define risk in this group. In practice, the only way this will ever be further clarified is by robust pharmacovigilance employed during large-scale MDA implementation programs.
In this study, all patient QTc measurements after three courses of DHA-PQP treatment were within approved safety margins outlined by U.S. drug regulatory institutions. The data do not suggest that the known risk of QT prolongation increases cumulatively with repeated monthly courses out to the 3rd course. However, the observation of slightly increased QT prolongation after two courses of treatment requires further investigation. At this stage, data from this study, together with the lack of reported adverse cardiac events in repeated-course MDA intervention programs elsewhere (5, 23), suggest that DHA-PQP can be used safely as MDA delivered using conventional dosing in up to 3 monthly rounds as a tool for malaria elimination. Cautions need to be articulated in relation to the antimalarial major drawbacks. First, multidrug resistance could reduce the impact; therefore, monitoring the drug’s efficacy will be required (24). Second, the three daily doses required for each round of MDA result in a major logistic burden and stretch the tolerance of the target population. Nevertheless, the absence of a single-dose antimalarial with safety data for repeated dosing support DHA-PQP as one of the best available options for MDA.
MATERIALS AND METHODS
Participants and study setting.
From 21 September to 21 December 2015, we conducted a prospective single-arm intervention study with healthy volunteers who resided in Lihir Island, New Ireland Province, Papua New Guinea (PNG). Eligible participants were male or female individuals age 3 to 60 years with good general health as determined by medical history, physical examination, baseline electrocardiographs, and laboratory tests. Participants were excluded if they (i) had a QT interval (adjusted using Fridericia’s correction [QTcF]) greater than 450 ms or clinically significant abnormalities of rhythm at screening, (ii) had a known history of additional risk factors for TdP, (iii) had a family history of long-QT syndrome or sudden cardiac death, (iv) were using concomitant medications known to prolong the QT/QTc interval, or (v) had a history of relevant allergic reactions. All female participants in reproductive age were tested for pregnancy (urinary beta-human chorionic gonadotropin [βHCG] dipstick) and excluded if they were in the first trimester.
The study protocol was approved by the PNG Institute of Medical Research institutional review board (number 15.01), the PNG Ministry of Health Medical Research Advisory Committee (number 15.14), and the ethics committee of Barcelona’s Hospital Clinic in Spain (HCB/2014/0424). Written informed consent was obtained from all adult participants or, for children, from parents or guardians.
Study procedures.
We recruited a nonprobabilistic convenience sample of healthy volunteers through group presentations by trained staff in 11 local communities, followed by one-to-one interviews. The volunteers were admitted at the hospital for a period of 72 h to facilitate drug administration under direct observation and fasting, as well as measurement of ECG traces. Arterakine (Pharbaco Central Pharmaceutical, Vietnam), registered product in PNG by the national authorities, was given daily for 3 days (i.e., at times 0, 24, and 48 h) according to the dosing schedule of the PNG National Malaria Treatment Protocol, DHA-PQP at 2.1/17.1 mg/kg of body weight (25). Patients fasted for the 3 h before and after each DHA-PQP dose.
Detailed physical examination, routine clinical laboratory tests, pregnancy test for women of reproductive age, a rapid diagnostic test (RDT) for malaria, and a malaria blood slide were performed at baseline (time 0 h) prior to treatment. Participants were assessed for adverse events (AEs) with a structured questionnaire and examination every 8 h throughout day 3 (i.e., at time 48, 56, 64, and 72 h) of each course. Examination included measurement of blood pressure, pulse, respiratory rate, cardiac auscultation, respiratory auscultation, and abdominal palpation for all participants. Blood samples to analyze drug levels were collected at 52 h (4 h after third dose) of each administration course.
Electrocardiography and electrocardiographic endpoints.
Twelve-lead ECG readings were conducted using an ELI 150 cardiograph at a speed of 25 mm/s at predose (at time 0 h, in triplicate), immediately prior to administration of the third dose (at time 48 h, single trace), and 4 h after the third dose (at time 52 h, in triplicate). For ECGs taken in triplicate, parameter measurements for the final analysis were based on the arithmetic mean of measurements from the 3 readings. Participants were discharged 24 h after the last dose of treatment (at time 72 h). In addition, ECGs were conducted at 7 days after the start of treatment and before (at time 0 h) the following treatment course. The same procedure was repeated for the second and the third monthly treatment courses. Throughout the study period, ECG readings were conducted at 12 time points over the 63-day follow-up period: day 0 predose (0 hcourse1), day 2 predose (48 hcourse1), day 2 postdose (52 hcourse1), day 7 (7 dayscourse1), day 28 predose (0 hcourse2), day 30 predose (48 hcourse2), day 30 postdose (52 hcourse2), day 35 (7 dayscourse2), day 56 predose (0 hcourse3), day 58 predose (48 hcourse3), day 58 postdose (52 hcourse3), and day 63 (7 dayscourse3).
The QT interval (i.e., distance from the Q wave to the end of the T wave) was corrected for heart rate using Fridericia’s correction formula (QTcF). This was defined as the measured QT interval divided by the cube root of the RR interval. The autocalculated QTcF measurements were manually verified by the study clinician for safety purposes. All ECG results were electronically transferred to a cardiac core lab (Banook/Cardiabase, France) where independent and centralized interpretation of the tracings were repeated by a certified cardiologist blinded to each participant’s details. The following parameters were obtained: RR (in milliseconds), HR (beats per minute), PR (in milliseconds), QRS (in milliseconds), QTcF (in milliseconds), and ΔHR (beats per minute), ΔPR (in milliseconds), ΔQRS (in milliseconds), ΔQTcF (in milliseconds) by comparison with baseline ECG reading (time 0 h) for each course of treatment.
A data safety monitoring board (DSMB) was established to which the site clinician (PM) was responsible for reporting any AE classified as either adverse events of special interest (AESIs) or severe adverse events (SAEs) according to whether they met prespecified criteria. We defined AESI as QTcF prolongation from baseline of >60 ms, QTcF at any time >450 ms, T-wave morphologic changes during therapy, bundle branch block, or any new arrhythmia. We defined cardiologic SAEs as a QTcF of >500 ms (sensitivity is 94% and specificity 97% for prediction of malignant arrhythmia in overdose schedules), any malignant ventricular arrhythmia (e.g., TdP), or any episode of sudden death (26). We established a predetermined threshold for study cessation as the occurrence of three episodes meeting any of our criteria for a cardiologic SAE.
Statistical analysis.
The primary endpoint of the study was ΔQTcF, calculated as the difference between QTcF measured at the 0-h recording to 52 h of each of the three courses. Secondary endpoints were (i) occurrences of AESIs and SAEs and (ii) QTcF resolution. For analysis of the primary endpoint, we estimated the risk difference and two-sided 95% CIs in ΔQTcF between the ECG reading at 52 hcourse3 and the ECG reading at 52 hcourse1 using a paired t test for comparisons. For analysis of the secondary endpoints, we estimated the risk difference and two-sided 95% CI for the difference in QTcF between the ECG reading at 0 hcourse3 and the ECG reading at 0 hcourse1, and we also looked at the difference between the ECG readings at 7 dayscourse3 and the ECG reading at 7 dayscourse1. We counted and summarized the number of AESIs, and we used the McNemar test to compare the difference in occurrence of AESIs between the third and first courses of treatment. Data analysis was performed using the Stata 15.1 software (Stata Corporation, College Station, TX, USA).
We calculated that 73 individuals would be required for the primary analysis to estimate a 5-ms difference in mean QTcF prolongation between the first and third treatment courses (assuming a prolongation in mean QTcF of 20 ms for the first month and 25 ms for the third month), with a confidence level of 95% and a power of 80%. Assuming that 10% of participants would be lost to follow-up, the target recruitment was 82 individuals.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge all the participants in the study and the Lihirian communities for the acceptance and collaboration in this research. We thank Anitua Mining Services and Newcrest Mining Limited for their support with the logistics in Lihir Island and Medicines for Malaria venture (MMV) for the research grant provided to ISGlobal as part of its collaborative agreement with Newcrest to support the Lihir Malaria Elimination Programme. We acknowledge International SOS and the staff at the Lihir Medical Centre for their work and support during the study, especially the Public Health Department and the Laboratory Department.
ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. CISM is supported by the Spanish Agency of International Cooperation (AECID). Q.B. is member of the WHO Malaria Treatment Guidelines Group. This group produces global guidance on the treatment of malaria, and this includes decisions about pyronaridine-artesunate. The views expressed by the authors are personal opinions and do not represent the recommendations of the WHO. The other authors declare no conflicts of interest.
O.M. and Q.B. conceived the study and developed the analysis plan. P.M.-M. and R.I. conducted the study, including patient enrollment and data collection. H.A. and K.P. conducted the laboratory tests. P.M.-M. and S.S. conducted the statistical analyses. O.M., P.M.-M. and Q.B. prepared the first draft of the article with important intellectual input and revisions from R.I., M.L., L.R., H.K., L.M., and B.M. All authors approved the final version of the article.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01153-18.
REFERENCES
- 1.WHO. 2015. Recommendations on the role of mass drug administration, mass screening and treatment, and focal screening and treatment for malaria. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.WHO. 2015. Guidelines for the treatment of malaria, 3rd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Eastman RT, Fidock DA. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutman J, Kovacs S, Dorsey G, Stergachis A, Ter Kuile FO. 2017. Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis. Lancet Infect Dis 17:184–193. doi: 10.1016/S1473-3099(16)30378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lwin KM, Phyo AP, Tarning J, Hanpithakpong W, Ashley EA, Lee SJ, Cheah P, Singhasivanon P, White NJ, Lindegardh N, Nosten F. 2012. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother 56:1571–1577. doi: 10.1128/AAC.05877-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, Muhindo MK, Osterbauer B, Aweeka FT, Huang L, Achan J, Havlir DV, Rosenthal PJ, Kamya MR, Dorsey G. 2014. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 11:e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini-Fuhrer I, Fofana B, Traore A, Diallo N, Diakite H, Togo AH, Koumare S, Keita M, Camara D, Somé AF, Coulibaly AS, Traore OB, Dama S, Goita S, Djimde M, Bamadio A, Dara N, Maiga H, Sidibe B, Dao F, Coulibaly M, Alhousseini ML, Niangaly H, Sangare B, Diarra M, Coumare S, Kabore MJT, Ouattara SM, Barry A, Kargougou D, Diarra A, Henry N, Soré H, Bougouma EC, Thera I, Compaore YD, Sutherland CJ, Sylla MM, Nikiema F, Diallo MS, Dicko A, Picot S, Borrmann S, Duparc S, Miller RM, Doumbo OK, et al. 2018. Pyronaridine–artesunate or dihydroartemisinin–piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial. Lancet 57:1378–1390. doi: 10.1016/S0140-6736(18)30291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EMA. 2011. Eurartesim. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001199/human_med_001450.jsp&mid=WC0b01ac058001d124. [Google Scholar]
- 9.EMA. Summary of product characteristics. Eurartesim. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001199/WC500118113.pdf. [Google Scholar]
- 10.Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, Hofmann NE, Kinboro B, Waltmann A, Brewster J, Lorry L, Tarongka N, Samol L, Silkey M, Bassat Q, Siba PM, Schofield L, Felger I, Mueller I. 2015. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med 12:e1001891. doi: 10.1371/journal.pmed.1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA. 2005. Guidance for industry. E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non antiarrhythmic drugs. U.S. Department of Health and Human Services, Food and Drug Administration, Rockville, MD. [Google Scholar]
- 12.White NJ, Looareesuwan S, Warrell DA. 1983. Quinine and quinidine: a comparison of EKG effects during the treatment of malaria. J Cardiovasc Pharmacol 5:173–175. doi: 10.1097/00005344-198303000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Karbwang J, Davis TM, Looareesuwan S, Molunto P, Bunnag D, White NJ. 1993. A comparison of the pharmacokinetic and pharmacodynamic properties of quinine and quinidine in healthy Thai males. Br J Clin Pharmacol 35:265–271. [PMC free article] [PubMed] [Google Scholar]
- 14.vn Seidlein L, Jaffar S, Greenwood B. 1997. Prolongation of the QTc interval in African children treated for falciparum malaria. Am J Trop Med Hyg 56:494–497. doi: 10.4269/ajtmh.1997.56.494. [DOI] [PubMed] [Google Scholar]
- 15.Karunajeewa H, Lim C, Hung TY, Ilett KF, Denis MB, Socheat D, Davis TM. 2004. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br J Clin Pharmacol 57:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mytton OT, Ashley EA, Peto L, Price RN, La Y, Hae R, Singhasivanon P, White NJ, Nosten F. 2007. Electrocardiographic safety evaluation of dihydroartemisinin piperaquine in the treatment of uncomplicated falciparum malaria. Am J Trop Med Hyg 77:447–450. doi: 10.4269/ajtmh.2007.77.447. [DOI] [PubMed] [Google Scholar]
- 17.Baiden R, Oduro A, Halidou T, Gyapong M, Sie A, Macete E, Abdulla S, Owusu-Agyei S, Mulokozi A, Adjei A, Sevene E, Compaore G, Valea I, Osei I, Yawson A, Adjuik M, Akparibo R, Ogutu B, Upunda GL, Smith P, Binka F. 2015. Prospective observational study to evaluate the clinical safety of the fixed-dose artemisinin-based combination Eurartesim (dihydroartemisinin/piperaquine), in public health facilities in Burkina Faso, Mozambique, Ghana, and Tanzania. Malar J 14:160. doi: 10.1186/s12936-015-0664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landier J, Kajeechiwa L, Thwin MM, Parker DM, Chaumeau V, Wiladphaingern J, Imwong M, Miotto O, Patumrat K, Duanguppama J, Cerqueira D, Malleret B, Renia L, Nosten S, von Seidlein L, Ling C, Proux S, Corbel V, Simpson JA, Dondorp AM, White NJ, Nosten FH. 2017. Safety and effectiveness of mass drug administration to accelerate elimination of artemisinin-resistant falciparum malaria: a pilot trial in four villages of Eastern Myanmar. Wellcome Open Res doi: 10.12688/wellcomeopenres.12240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisele TP, Bennett A, Silumbe K, Finn TP, Chalwe V, Kamuliwo M, Hamainza B, Moonga H, Kooma E, Chizema Kawesha E, Yukich J, Keating J, Porter T, Conner RO, Earle D, Steketee RW, Miller JM. 2016. Short-term impact of mass drug administration with dihydroartemisinin plus piperaquine on malaria in Southern Province Zambia: a cluster-randomized controlled trial. J Infect Dis 214:1831–1839. doi: 10.1093/infdis/jiw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng C, Huang B, Wang Q, Wu W, Zheng S, Zhang H, Li D, Feng D, Li G, Xue L, Yang T, Tuo F, Mohadji F, Su XZ, Xu Q, Wu Z, Lin L, Zhou J, Yan H, Bacar A, Said Abdallah K, Keke RA, Msa Mliva A, Mohamed M, Wang X, Huang S, Oithik F, Li XB, Lu F, Fay MP, Liu XH, Wellems TE, Song J. 2018. Large-scale artemisinin-piperaquine mass drug administration with or without primaquine dramatically reduces malaria in a highly endemic region of Africa. Clin Infect Dis doi: 10.1093/cid/ciy364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonsson MK, Vos MA, Duker G, Demolombe S, van Veen TA. 2010. Gender disparity in cardiac electrophysiology: implications for cardiac safety pharmacology. Pharmacol Ther 127:9–18. doi: 10.1016/j.pharmthera.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Moss AJ. 1993. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol 72:23b–25b. doi: 10.1016/0002-9149(93)90036-C. [DOI] [PubMed] [Google Scholar]
- 23.Song J, Socheat D, Tan B, Dara P, Deng C, Sokunthea S, Seila S, Ou F, Jian H, Li G. 2010. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar J 9:57. doi: 10.1186/1475-2875-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, Sam B, Dek D, Try V, Amato R, Blessborn D, Song L, Tullo GS, Fay MP, Anderson JM, Tarning J, Fairhurst RM. 2016. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Department of Health. 2009. National malaria treatment protocol. National Department of Health Papua New Guinea, Port Moresby, Papua New Guinea. [Google Scholar]
- 26.WHO. 2016. Evidence Review Group meeting on cardiotoxicity of antimalarials. World Health Organization, 13 to 14 October 2016, Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.