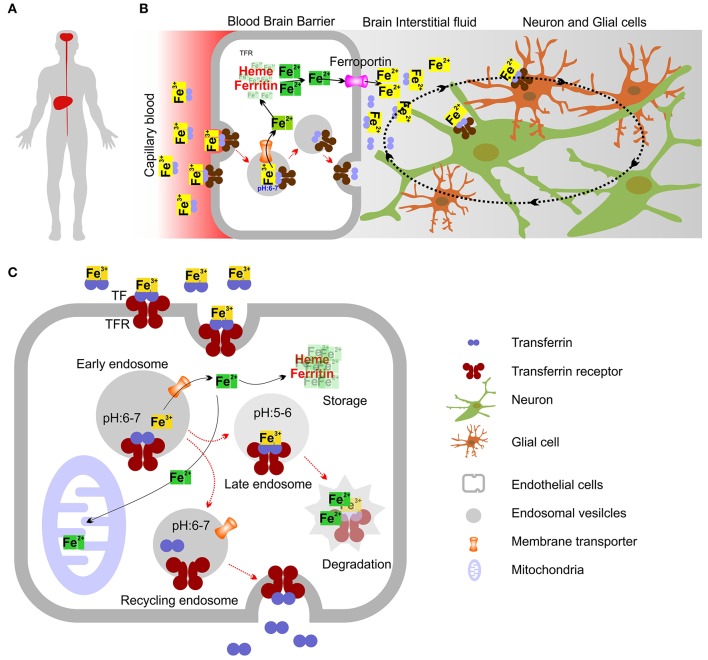
Figure 11.
Model showing intracellular iron transport and transport across Blood-Brain Barrier (BBB). (A) Diagrammatic representation showing Liver and brain as 2 organs in human involved in the production of TF. (B) Mechanism showing how iron crosses the BBB. Holo-TF circulates through the brain capillaries and comes in contact with the luminal TFR which then internalize TF bound iron. The iron exporter ferroportin may allow iron to cross the abluminal membrane of the endothelial cell to enter the interstitial fluid. Most TF in the brain interstitial fluid is synthesized and secreted by oligodendrocytes as Apo-TF. Neurons and glia may acquire most iron through the TFR and holo-TF that is present in the interstitial fluid and cerebrospinal fluid. (C) Molecular mechanism showing intracellular iron transport. Most cellular uptake of ferric iron (Fe3+) occurs via receptor-mediated endocytosis of TF. Fe3+ ions form complexes with the high-affinity iron binding TF, which then binds to TFR. After endocytosis of TFR, the acidic environment of the early endosomes triggers the release of Fe3+ from the TF-TFR complex, which is recycled to the plasma membrane via recycling endosomes. Ferric reductases localized to the endosome reduce Fe3+ (ferric) to its Fe2+ (ferrous) form before Fe2+ is released into the cytosol by the divalent metal transporter-1 (DMT1) in an H+-dependent manner. Fe3+ may also be sorted into late endosomes (LE) and lysosomes (LY), where it is reduced to Fe2+ by ferric reductases. In LE and LY, Fe2+ can also be released by other endo-lysosomal iron release channels/transporters. Iron can then bind to the chaperones that donate iron to specific target proteins (not shown) (Philpott, 2012) or enter mitochondria through the dedicated mitochondrial iron transporters (Chen et al., 2009), where it is used for the synthesis of iron–sulfur clusters and haem (Rouault, 2013). Iron can also be stored in cytosolic proteins such as ferritin, which can sequester up to 4,500 iron atoms (Arosio et al., 2009). Ferritin sequestration of iron prevents free iron from reaching high concentrations in the cytosolic and nuclear compartments (Arosio et al., 2009). Degradation of ferritin in lysosomes leads to the formation of disorganized iron-rich deposits known as haemosiderin (not shown) (Cohen et al., 2010).