Table 1.
1H-NMR, 13C-NMR and HMBC correlations of 4a(CDCl3).
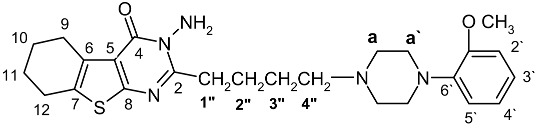
| No | 13-NMR(DEPT) | 1H-NMR | HMBC | |
|---|---|---|---|---|
| δC | δH | 2J | 3J | |
| 1 | - | |||
| 2 | 156.97 (C) | |||
| 3 | - | 4.96 (NH2) | C-2 | |
| 4 | 161.57 (C) | |||
| 5 | 158.18 (C) | |||
| 6 | 130.91 (C) | |||
| 7 | 119.87 (C) | |||
| 8 | 141.34 (C) | |||
| 9 | 25.16 (CH2) | 2.71 | C-6, 10 | C-5, 7, 11 |
| 10 | 22.24 (CH2) | 1.83 | C-9, 11 | C-6, 12 |
| 11 | 22.95 (CH2) | 1.83 | C-10, 12 | C-7, 9 |
| 12 | 25.39 (CH2) | 2.95 | C-7, 11 | C-6, 10 |
| 1′ | 152.29 (C) | |||
| 2′ | 111.31 (CH) | 6.83(d)* | C-1′, 3′ | C-4′, 6′ |
| 3′ | 121.01 (CH) | 6.88-6.93 | C-2′, 4′ | C-1′, 5′ |
| 4′ | 122.84 (CH) | 6.97(t)* | C-3′, 5′ | C-2′, 6′ |
| 5′ | 118.22 (CH) | 6.88-6.93 | C-4′, 6′ | C-1′, 3′ |
| 6′ | 133.05 (C) | |||
| O-CH3 | 55.34 (CH3) | 3.84 | C-1′ | |
| 1ʺ | 33.99(CH2) | 2.98(t) | C-2ʺ, 2 | C-3ʺ |
| 2ʺ | 26.25(CH2) | 1.65 | C-1ʺ, 3ʺ | C-2, 4ʺ |
| 3ʺ | 24.65(CH2) | 1.83 | C-2ʺ, 4ʺ | C-1ʺ |
| 4ʺ | 58.32(CH2) | 2.47(t) | C-3ʺ | C-a, 2ʺ |
| a | 53.75(2CH2) | 2.66(2 CH2) | C-a′ | C-4ʺ |
| a′ | 50.33(2CH2) | 3.09(2 CH2) | C-a | C-6′ |
* J = 7.0 Hz.
