Table 1.
Approved anti-HBV drugs.
| Molecule | Structure | Brand name | Mechanism | Company | Year of FDA approval |
|---|---|---|---|---|---|
| Lamivudine | 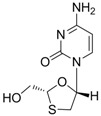 |
Zeffix, Heptovir, Epivir, and Epivir-HBV | Nucleoside analogue/RT Inhibitor | GlaxoSmithKline | 1998 (for adults) and 2000 (for children) in US |
| Adefovir | 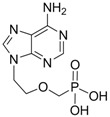 |
Preveon and Hepsera | Nucleotide analogue/RT Inhibitor | Gilead | 2002 (US) |
| Entecavir | 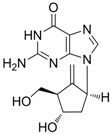 |
Baraclude | Nucleoside analogue/RT Inhibitor | Bristol Meyers Squibb | 2005 (US) |
| Telbivudine | 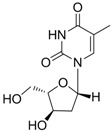 |
Sebivo (Europe) Tyzeka (US) | Nucleoside analogue/RT Inhibitor | Idenix, Novartis | 2006 (US) |
| Clevudine | 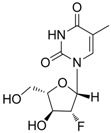 |
Levovir and Revovir | Nucleoside analogue/RT Inhibitor. | Bukwang Pharm | 2006 (KOREA) |
| Tenofovir | 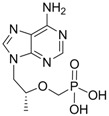 |
Viread | Nucleotide analogue/RT Inhibitor | Gilead | 2008 (US) |
