Table 2.
Preparation of benzofuran 19.
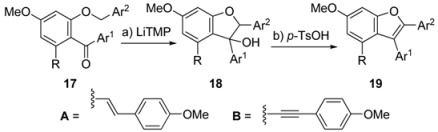 | ||||
|---|---|---|---|---|
| Entry | R | Ar1 | Ar2 | 19 Yield (%)a |
| 1(a) | H | C6H5 | C6H5 | 83 |
| 2(b) | A | 3,5-(MeO)2C6H3 | C6H5 | 71 |
| 3(c) | A | 3,5-(MeO)2C6H3 | 4-(Br)C6H4 | 0 |
| 4(d) | A | 3,5-(MeO)2C6H3 | 4-(MeO)C6H4 | 87 |
| 5(e) | A | 3,5-(MeO)2C6H3 | 2-furyl | 38 |
| 6(f) | A | C6H5 | 4-(MeO)C6H4 | 80 |
| 7(g) | A | 3,4,5-(MeO)3C6H2 | 4-(MeO)C6H4 | 85 |
| 8(h) | A | 3,4-(MeO)2C6H3 | 4-(MeO)C6H4 | 81 |
| 9(i) | B | 3,5-(MeO)2C6H3 | 4-(MeO)C6H4 | 85 |
Reagents and conditions: (a) LiTMP (0.5 M in THF, 5 equiv), THF, 0 °C, 2 h; (b) p-TsOH•H2O (1.0 equiv), CH2Cl2, 23 °C, 1 h. aYields refer to chromatographically and spectroscopically homogeneous material. LiTMP = Lithium 2,2,6,6-tetramethylpiperidide; p-TsOH = toluenesulfonic acid.
