Table 3.
Friedel−Crafts type cyclization of benzofurans 20
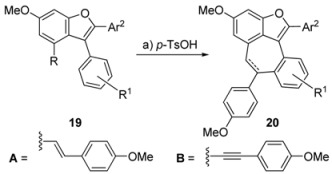 | ||||
|---|---|---|---|---|
| Entry | R | R1 | Ar2 | 20 Yield (%) a |
| 1(a) | A | 3,5-(MeO)2 | C6H5 | 95 |
| 2(b) | A | 3,5-(MeO)2 | 4-(MeO)C6H4 | 90 |
| 3(c) | A | 3,5-(MeO)2 | 2-furyl | 0 |
| 4(d) | A | H | 4-(MeO)C6H4 | 0 |
| 5(e) | A | 3,4,5-(MeO)3 | 4-(MeO)C6H4 | 92 |
| 6(f) | A | 3,4-(MeO)2 | 4-(MeO)C6H4 | 90 |
| 7(g) | B | 3,4-(MeO)2 | 4-(MeO)C6H4 | 95 |
Reagents and conditions: (a) p-TsOH•H2O (3.0 equiv), CH2Cl2, 40 °C, 8 h. aYields refer to chromatographically and spectroscopically homogeneous material.
