Abstract
N-acetylcysteine (NAC) is a thiol-containing antioxidant that modulates the intracellular redox state. NAC can scavenge reactive oxygen species (ROS) and maintain reduced glutathione (GSH) levels, in order to protect cardiomyocytes from oxidative stress. The present study aimed to determine whether NAC protects cardiomyocytes from oxidative damage by regulating the redox status of intracellular antioxidant proteins. The results revealed that NAC pretreatment increased cell viability and inhibited the activation of caspase-3, -8 and -9 during hydrogen peroxide (H2O2)-induced oxidative stress in H9c2 cells. Furthermore, decreased ROS levels, and increased total and reduced GSH levels were detected in response to NAC pretreatment. Non-reducing redox western blotting was performed to detect the redox status of intracellular antioxidant proteins, including thioredoxin 1 (Trx1), peroxiredoxin 1 (Prx1), GSH reductase (GSR), and phosphatase and tensin homolog (PTEN). The results revealed that the reduced form of Trx1 was markedly increased, and the oxidized forms of Prx1, GSR and PTEN were decreased following NAC pretreatment. Furthermore, NAC pretreatment decreased H2O2-induced phosphorylation of apoptosis signal-regulating kinase 1, which depends on the redox state of Trx1, and increased H2O2-induced phosphorylation of protein kinase B, which is essential to cell survival. To the best of our knowledge, the present study is the first to reveal that NAC pretreatment may alleviate oxidation of intracellular antioxidant proteins to inhibit oxidative stress-induced cardiomyocyte apoptosis.
Keywords: oxidative stress, redox state, N-acetylcysteine, thioredoxin 1, peroxiredoxin 1, glutathione reductase
Introduction
Myocardial ischemia-reperfusion injury is considered a more severe form of myocardial injury than ischemia alone, due to aggravated oxidative stress (1). Oxidative stress refers to an imbalance between pro-oxidants and antioxidants (2), and is associated with the pathogenesis of myocardial ischemia-reperfusion injury.
To control the cellular redox environment, cells contain two primary redox regulatory systems: The glutathione (GSH)/GSH reductase (GSR)/GSH peroxidase (GPx)/glutaredoxin (Grx) pathway, and the thioredoxin (Trx)/peroxiredoxin (Prx) pathway (3). These two major thiol-dependent antioxidant systems serve key roles in the defense against oxidative stress (4,5). Trx, Grx and Prx have been characterized as regulators of the intracellular redox state, and are increasingly being recognized for their specific roles in redox signaling. It has previously been reported that these antioxidants regulate cell survival and death through redox-dependent signal transduction pathways during oxidative stress (2). Therefore, the redox states of these antioxidants are critical for the fate of cells under oxidative conditions.
Maintenance of the GSH redox couple, GSH/GSH disulfide (GSSG), is achieved by recycling via the pentose phosphate pathway and GSH biosynthesis. N-acetylcysteine (NAC), which is a precursor of GSH, is widely used and has attracted great interest as a thiol-containing antioxidant and modulator of the intracellular redox state (6). In addition, it has been demonstrated that repletion of GSH levels through NAC protects against oxidative stress-induced cell death though scavenging of free radicals (7). Furthermore, NAC can inhibit oxidative stress-induced apoptosis of cardiomyo-cytes by decreasing caspase-3 activity (8,9). Wang et al (10), revealed that NAC and allopurinol reduce myocardial ischemia-reperfusion injury in diabetes by activating the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and Janus kinase 2/signal transducer and activator of transcription 3 pathways. Kumar and Sitasawad demonstrated that NAC prevents glucose/glucose oxidase-induced oxidative stress by normalizing mitochondrial membrane potential, inhibiting cytochrome c release, increasing B-cell lymphoma 2 (Bcl-2) expression, decreasing Bcl-2-associated X protein expression and activating procaspase-9 in H9c2 cells (11).
The present study investigated whether NAC protects cardiomyocytes from oxidative damage by regulating the redox status of intracellular antioxidant proteins. The results revealed that NAC pretreatment alleviated the oxidation of intracellular antioxidants, such as Trx1, Prx1 and GSR, in order to protect cardiomyocytes from oxidative stress-induced apoptosis.
Materials and methods
Reagents and antibodies
NAC (cat. no. A9165), N-ethylmaleimide (NEM; cat. no. E3876) and trichlo-roacetic acid (cat. no. T0699) were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany, China). Chloromethyl-dichlorofluorescein diacetate (CM-H2DCFDA; cat. no. C6827) was purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Anti-Prx1 (cat. no. 8499), anti-Trx1 (cat. no. 2429), anti-PTEN (cat. no. 9552), anti-green fluorescent protein (GFP; cat. no. 2956S), anti-apoptosis signal-regulating kinase 1 (ASK1; cat. no. 8662S), anti-Akt (cat. no. 9272S) and anti-phosphorylated (p-ASK1; Thr845) (cat. no. 3765) and p-Akt (Thr308) (cat. no. 9275) antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA), and anti-GSR (cat. no. sc-133245) was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-GAPDH (cat. no. BM3874) was purchased from Wuhan Boster Biological Technology Ltd. (Wuhan, Hubei, China). Caspase-3 (cat. no. K106), caspase-8 (cat. no. K113) and caspase-9 (cat. no. K119) assay kits were purchased from BioVision, Inc. (Milpitas, CA, USA).
Cell culture
H9c2 cells (cat. no. CRL-1446; American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. Subsequently, the cells were seeded at varying densities in 6-well plates or 6-cm dishes, incubated for 24 h, and then treated with NAC or H2O2 for the desired time periods.
MTT assay for cell viability
The MTT assay is a standard method used to assess cell viability. Briefly, H9c2 cells were washed twice with PBS, detached with 0.25% trypsin and made into a single cell suspension. Cells were seeded into 96-well microtiter plates at a density of 3-5×103 cells/well in 200 µl culture medium. Following incubation with 4 mM NAC for 1 h at 37°C, the cells were treated with 0.75 mM H2O2 and were incubated for various durations. Subsequently, 100 µl MTT solution (0.5 mg/ml) was added to each well and the cells were incubated for 4 h at 37°C, after which, 150 µl dimethyl sulfoxide was added to each well. The absorbance was measured at 570 nm, and the values were used to calculate the relative ratio of viable cells to total cells.
Apoptosis assay
H9c2 cells were pretreated with 4 mM NAC for 1 h, ad were then treated with 0.75 mM H2O2 for 0, 12 and 24 h. A total of 2-3×104 cells were collected in a tube and were washed twice with PBS. Subsequently, the cells were centrifuged at 10,000 × g for 10 min at 4°C; the supernatant was removed and the cell pellets were resus-pended in 500 µl binding buffer containing 5 µl Annexin V-fluorescein isothiocyanate and 5 µl propidium iodide (cat. no. 556547; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA). Following incubation at room temperature in the dark for 15 min, cell apoptosis was assessed by fluorescence-activated cell sorting (Beckman Coulter, Inc., Brea, CA, USA).
GSH and GSSG measurements
The levels of GSH and GSSG were measured using a GSH assay kit (cat. no. 703002; Cayman Chemical Company, Ann Arbor, MI, USA), which employs a spectrophotometric GSR recycling assay. H9c2 cells were pretreated with 4 mM NAC for 1 h, and were then treated with 0.75 mM H2O2 for 0, 15 and 30 min. A total of 3-5×106 cells were washed twice with chilled PBS, scraped into cold buffer containing 0.2 M 2-(N-morpholino) ethanesulfonic acid, 50 mM phosphate and 1 mM EDTA (pH 6.0), sonicated (20-25 kHz) on ice twice for 20 sec, and centrifuged at 10,000 × g for 15 min at 4°C. The supernatant was removed and deproteinated for analysis of total GSH and GSSG, according to the manufacturer’s protocol. Reduced GSH levels were calculated from the total GSH and GSSG levels. All determinations were normalized to protein content, as determined using a bicinchoninic acid (BCA) protein assay kit (cat. no. 23227; Thermo Fisher Scientific, Inc.). The absorbance was measured at 405 nm using a plate reader at 5-min intervals for 30 min.
ROS measurement
ROS production was measured using the cell-permeable probe, CM-H2DCFDA. Cells were plated 24 h prior to analysis in 6-well plates. Cells were incubated with CM-H2DCFDA at a concentration of 10 µM for 30 min at 37°C. Subsequently, the cells were washed twice with PBS and incubated with 4 mM NAC for 1 h, followed by treatment with 0.75 mM H2O2 for 15 and 30 min. Fluorescence was quantified by flow cytometry (Gallios; Beckman Coulter, Inc.) with excitation and emission wavelengths of 485 and 530 nm, respectively.
Caspase-3, -8 and -9 activity assay
Caspase-3, -8 and -9 activities were measured using caspase colorimetric assay kits (BioVision, Inc.). Briefly, total cellular protein levels were quantified using the bicinchoninic acid protein assay kit (cat. no. 23227; Thermo Fisher Scientific, Inc.) and reacted with the corresponding substrates: DEVD-pNA, IETD-pNA and LEHD-pNA. Caspase-3, -8 and -9 activities were subsequently measured as the optical density of the cleaved substrate, ρNA, at 405 nm using a microplate reader (ELX-800; Bio-Tek Instruments, Inc., Winooski, VT, USA).
NEM-alkylated redox western blotting
For mitochondrial reduction-oxidation-sensitive green fluorescent protein (mito-roGFP) analysis, 3-5×106 H9c2 cells were transiently transfected with mito-roGFP (provided by Dr S. James Remington, University of Oregon, Eugene, OR, USA) using Lipofectamine® 2000 (cat. no. 11668019; Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at 37°C.
H9c2 cells (3-5×106) were treated with 0.75 mM H2O2 for 0, 0.25, 0.5, 1, 2 and 3 h and NEM-alkylated redox western blotting was performed to detect the redox states of GSR and PTEN. Alternatively, following incubation with 4 mM NAC for 1 h at 37°C, the cells (3-5×106) were treated with 0.75 mM H2O2 for 0, 15 and 30 min, and NEM-alkylated redox western blotting was performed to detect the redox state of mito-GFP, Prx1, Trx1, GSR and PTEN. The present study analyzed the redox states of mito-GFP, Prx1, Trx1, GSR and PTEN using methods reported by Poynton and Hampton (12). Cells were washed twice with ice-cold PBS immediately after treatment. Subsequently, cells were precipitated with chilled trichloroacetic acid (10%) for 30 min at 4°C, centrifuged at 12,000 × g for 10 min and washed twice with 100% acetone. The protein pellets were dissolved in nonreducing buffer (100 mM Tris-HCl, pH 6.8; 2% SDS; and either 40 mM NEM for Trx, GSR, GFP and PTEN or 100 mM NEM for Prx1). Trx1 redox forms were separated by 17% non-reducing SDS-PAGE; Prx1, GSR and PTEN redox forms were separated by 15% non-reducing SDS-PAGE. For reducing SDS-PAGE, 5% dithiothreitol was added to the samples (30 or 50 µg protein was loaded onto gels) and the proteins were transferred onto polyvinylidene fluoride membranes. Subsequently, membranes were blocked with 5% dry milk in TBS-0.1% Tween-20 (TBST) for 1 h at room temperature, and were incubated with the GFP (1:1,000), GSR (1:1,000), Prx1 (1:1,000), Trx1 (1:1,000) and PTEN (1:1,000) antibodies diluted in TBST and 0.2% bovine serum albumin (cat. no. TS-38839; Thermo Fisher Scientific, Inc.) overnight at 4°C. After washing three times with PBS (15 min/wash), the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:5,000; cat no. BS13278; Bioworld Technology, Inc., St. Louis Park, MN, USA) for 1 h at room temperature. Bands were semi-quantified using ImageJ (1.48v) software (National Institutes of Health, Bethesda, MD, USA).
SDS-PAGE and immunoblotting
To detect ASK, p-ASK, Akt and p-Akt expression, cell lysates were extracted using 1X SDS buffer (0.5 M Tris-HCl, pH 6.8; 20% SDS; 10% glycerol). The concentration of the samples was determined using a BCA protein assay kit (cat. no. 23227; Thermo Fisher Scientific, Inc.). Subsequently, 30 or 50 µg protein was separated by 12% glycine SDS-PAGE and transferred onto polyvinylidene fluoride membranes. Subsequently, membranes were blocked with 5% dry milk in TBST for 1 h at room temperature, and were incubated with ASK (1:1,000), p-ASK (1:1,000), Akt (1:1,000), p-Akt (1:1,000), GAPDH (1:1,000) and GFP (1:1,000) antibodies diluted in TBST and 0.2% bovine serum albumin overnight at 4°C. The subsequent immunoblotting steps were conducted as aforementioned.
Statistical analysis
Each experiment was repeated three times. For western blotting, one representative image is shown in the figures. Results are presented as the means ± standard deviation. Statistical significance was assessed by one-way analysis of variance followed by Tukey’s multiple comparisons test using GraphPad Prism Version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
NAC pretreatment attenuates H2O2-induced cytotoxicity, and inhibits H2O2-induced activation of caspase-3, -8 and -9 in H9c2 cells
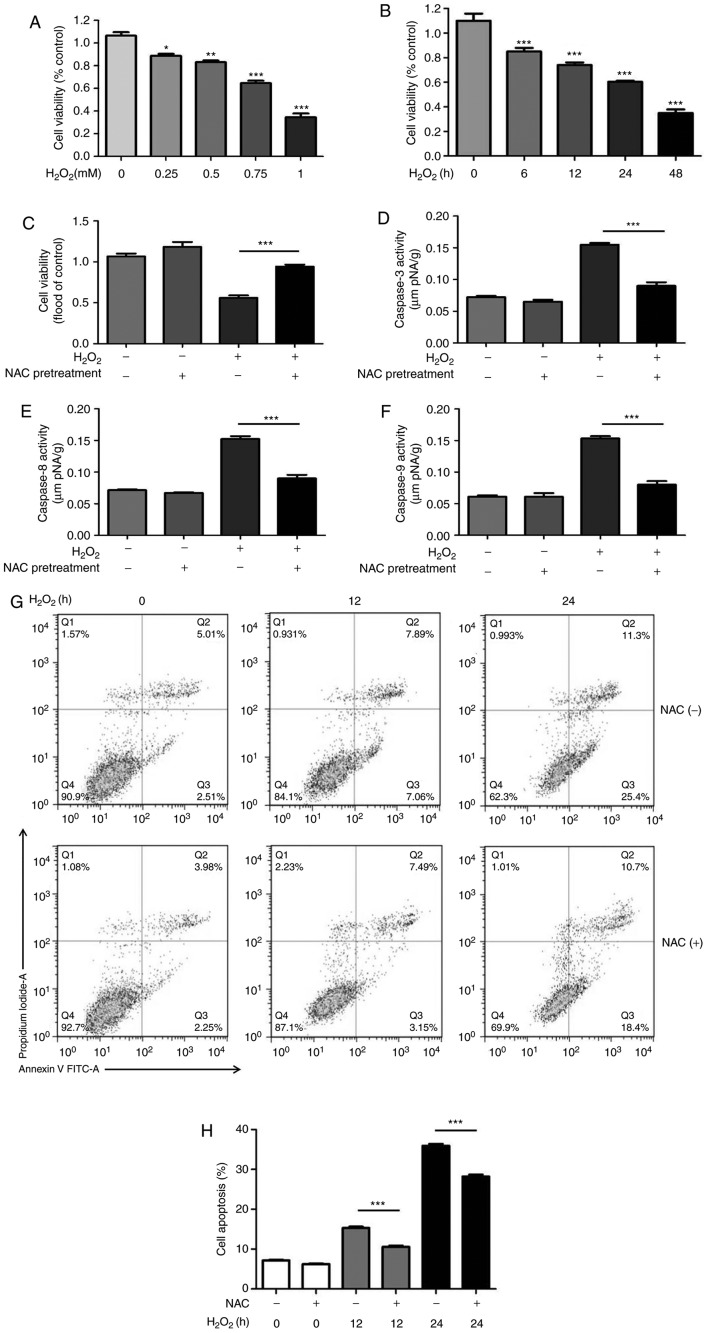
To investigate the effects of NAC pretreatment on H2O2-induced H9c2 cell death, cell viability was measured based on the uptake and reduction of MTT to an insoluble formazan dye. Compared with in the control group, the viability of H9c2 cells was significantly decreased by H2O2 in a dose- and time-dependent manner (Fig. 1A and B). Based on these results, 0.75 mM H2O2 was used in subsequent experiments. As shown in Fig. 1C, 4 mM NAC pretreatment for 1 h markedly enhanced cell viability during H2O2-induced oxidative injury. The dose of NAC was chosen based on previous in vitro studies (11,13).
Figure 1.
NAC pretreatment attenuates H2O2-induced cytotoxicity. (A) H9c2 cells were treated with various concentrations of H2O2 (0.25, 0.5, 0.75 or 1.0 mM) for 24 h. (B) H9c2 cells were treated with 0.75 mM H2O2 for 0, 6, 12, 24 and 48 h. MTT assay was used to measure cell viability. Data are presented as the means ± standard deviation (n=8 per group). (C) H9c2 cells were pretreated with 4 mM NAC for 1 h and were then incubated with 0.75 mM H2O2 for 24 h. MTT was used to measure cell viability. Data are presented as the means ± standard deviation (n=8). Enzymatic activities of (D) caspase-3, (E) caspase-8 and (F) caspase-9 were measured using a colorimetric assay. (G and H) H9c2 cells were pretreated with 4 mM NAC for 1 h and were then incubated with 0.75 mM H2O2 for the indicated times (0, 12 and 24 h), and flow cytometric analysis of cell apoptosis was conducted. (G) Representative plots of flow cytometric analysis are presented. (H) Results of flow cytometry indicated that NAC significantly reduced the percentage of apoptotic H9c2 cells. Data are presented as the means ± standard deviation (n=6). *P<0.05, **P<0.01, ***P<0.001. FITC, fluorescein isothiocyanate; H2O2, hydrogen peroxide; NAC, N-acetylcysteine.
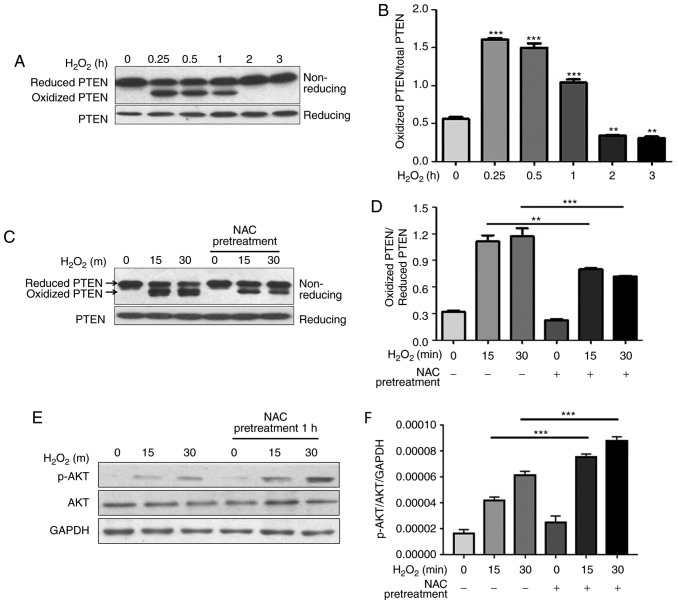
Figure 4.
NAC pretreatment decreases H2O2-induced oxidation of PTEN and increases phosphorylation of AKT. N-ethylmaleimide-alkylated redox western blotting was performed to detect the redox state of (A-D) PTEN. (B and D) Reduced and oxidized forms of PTEN were semi-quantified using ImageJ software. Data are presented as the means ± standard deviation (n=3). (E) Western blotting was performed to detect total and p-AKT. (F) p-AKT was semi-quantified using ImageJ software. Data are presented as the means ± standard deviation (n=3). **P<0.01, ***P<0.001 as indicated, or vs. 0 h H2O2 group. AKT, protein kinase B; H2O2, hydrogen peroxide; NAC, N-acetylcysteine; p, phosphorylated; PTEN, phosphatase and tensin homolog.
To determine whether NAC pretreatment suppresses H2O2-induced apoptosis, the activities of initiator (caspase-8 and -9) and effector caspases (caspase-3) were measured using colorimetric assays. The results revealed that NAC pretreatment decreased the activation of caspase-3, -8 and -9 induced by H2O2 (Fig. 1D-F). To further confirm whether NAC alleviates H2O2-induced H9c2 cell apoptosis, flow cytometric analysis was conducted. The results indicated that NAC significantly reduced the percentage of apoptotic H9c2 cells (Fig. 1G and H), thus suggesting that NAC pretreatment may protect H9c2 cell from H2O2-induced cell death.
NAC regulates the redox state of the cytoplasm and mitochondria in H9c2 cells
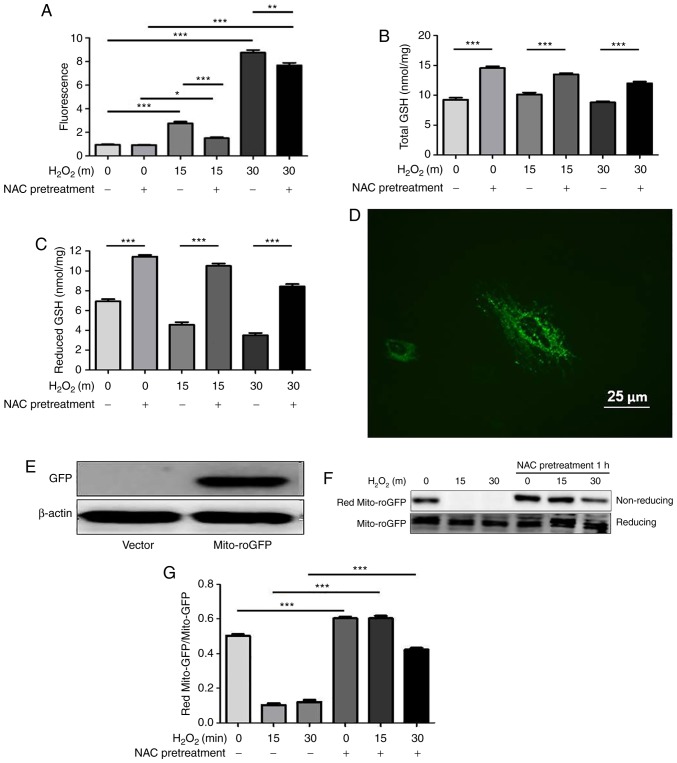
Increasing evidence has demonstrated that repletion of GSH levels through NAC protects against oxidative stress-induced cell death though the scavenging of free radicals. ROS levels were measured by flow cytometry using the CM-H2DCFDA dye. As shown in Fig. 2A, ROS levels in the control and NAC groups were low. Conversely, increased ROS levels were observed in the H2O2-stimulated cells, thus indicating that oxidative stress was induced, whereas these levels were significantly attenuated by NAC pretreatment. NAC is a precursor of GSH; in order to determine the effects of NAC pretreatment on intracellular redox state, total and reduced GSH levels were determined using a spectrophotometric GSR recycling assay. The results revealed that NAC pretreatment markedly increased the levels of total and reduced GSH (Fig. 2B and C).
Figure 2.
NAC pretreatment decreases levels of reactive oxygen species and increases GSH under oxidative conditions in H9c2 cells. (A) H9c2 cells were incubated with CM-H2DCFDA dye at a concentration of 10 µM for 30 min at 37°C. Cells were pretreated with 4 mM NAC for 1 h and were then treated with 0.75 mM H2O2 for the indicated durations (15 and 30 min). Fluorescence was quantified using flow cytometry with excitation and emission wavelengths of 485 and 530 nm, respectively. Values are presented as the means ± standard deviation (n=6). Intracellular (B) total and (C) reduced GSH levels were determined using a GSH reductase recycling assay. (D) Immunofluorescence of mito-roGFP (green) in H9c2 cells (magnification, x400). Scale bar, 25 µm. (E) Western blotting revealed the expression levels of mito-roGFP and β-actin. (F and G) H9c2 cells were transfected with mito-roGFP or vector for 48 h and were then treated with 0.75 mM H2O2 for the indicated times (15 and 30 min). Non-reducing western blotting was performed to detect the redox state of mito-roGFP. Data are presented as the means ± standard deviation (n=6). *P<0.05, **P<0.01, ***P<0.001. GFP, green fluorescent protein; GSH, glutathione; H2O2, hydrogen peroxide; mito-roGFP, mitochondrial redox-sensitive GFP; NAC, N-acetylcysteine.
To directly assess the redox state in mitochondria, mito-roGFP was used, as described in our previous study (13), whose fluorescent properties at two excitation wavelengths change in response to formation of an engineered disulfide bond. This method allows for dynamic monitoring of the subcellular redox status of GSH/GSSG within living cells. The present study transiently transfected mitochondrial mito-roGFP into H9c2 cells. As shown in Fig. 2D-G, redox western blotting revealed that NAC significantly increased the reduced forms of mito-roGFP in response to oxidative stress. These results suggested that NAC pretreatment may modify cytoplasmic and mitochondrial redox states.
NAC pretreatment attenuates H2O2-induced oxidation of Trx1, Prx1 and GSR
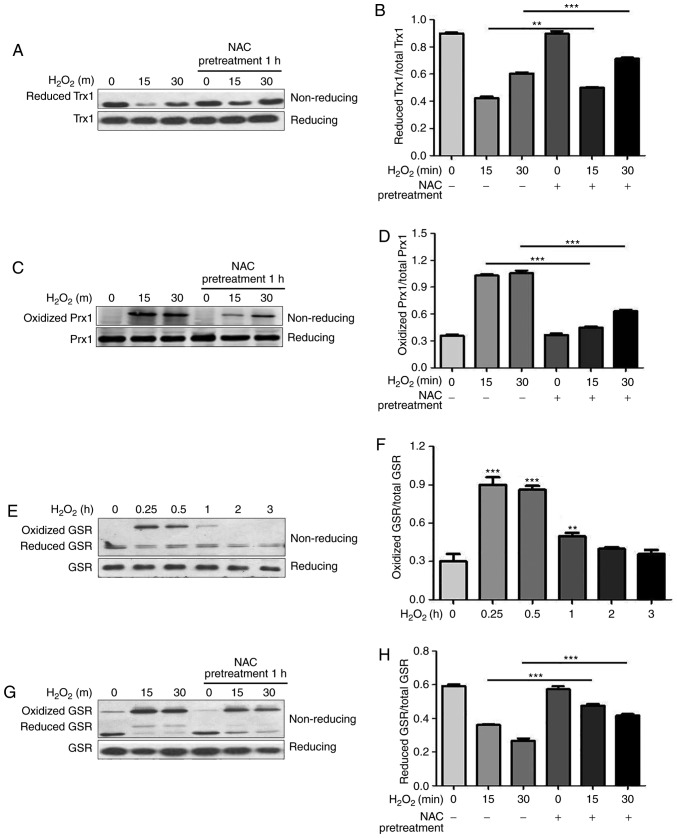
Trx and Prx are important thiol peroxidases that regulate intracellular ROS levels. Under oxidative stress, Prx or Trx oxidation products accumulate in cells. The accumulation of oxidized Trx/Prx may disrupt cellular redox homeostasis, promote apoptotic signaling pathways and determine the fate of cells (2). To determine the effects of NAC pretreatment on the redox state of antioxidant proteins in H9c2 cells under oxidative conditions, the redox states of Trx1 and Prx1 were measured via non-reducing SDS-PAGE. As reported in our previous study (13), NEM derivatization can be used to identify reduced Trx1 since it migrates faster than the oxidized form (Fig. 3A, upper panel). Following exogenous application of H2O2, the reduced form of Trx1 was decreased, thus suggesting an expected shift to the oxidized state, which could not be detected under this condition (Fig. 3A, upper panel). Conversely, there was no difference in total Trx1 levels following H2O2 treatment under reducing conditions (Fig. 3A, lower panel). As shown in Fig. 3A and B, reduced Trx1 was markedly increased by NAC pretreatment under oxidative conditions. Similar to previous study (13), the oxidized form of Prx1 was detected following H2O2 stimulation using the NEM derivatization method (Fig. 3C, upper panel), which migrated slower than the reduced form and disappeared under reducing conditions (Fig. 3C, lower panel). NAC pretreatment significantly attenuated oxidation of Prx1 by H2O2, as shown by a decrease in the oxidized form of Prx1 (Fig. 3C, upper panel and Fig. 3D).
Figure 3.
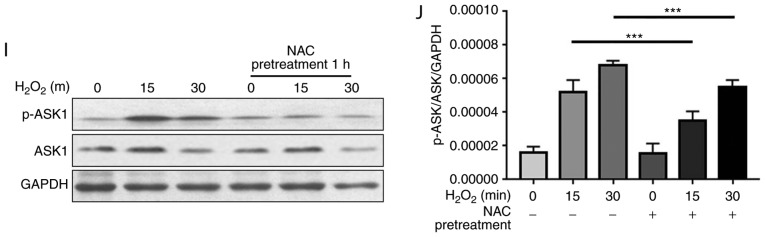
NAC pretreatment attenuates H2O2-induced oxidation of Trx1, Prx1 and GSR, and decreases phosphorylation of ASK1. N-ethylmaleimide-alkylated redox western blotting was performed to detect the redox states of (A and B) Trx1, (C and D) Prx1 and (E-H) GSR. For reducing SDS-PAGE, 5% dithiothreitol was added to the samples. (B, D, F and H) Reduced and oxidized forms of Trx1, Prx1 and GSR were semi-quantified using ImageJ software. Data are presented as the means ± standard deviation (n=3). (I) Western blotting was performed to detect total and p-ASK1. (J) p-ASK1 was semi-quantified using ImageJ software. Data are presented as the means ± standard deviation (n=3). **P<0.01, ***P<0.001 as indicated, or vs. the 0 h H2O2 group. ASK1, apoptosis signal-regulating kinase 1; GSR, glutathione reductase; H2O2, hydrogen peroxide; NAC, N-acetylcysteine; p, phosphorylated; Prx1, peroxiredoxin 1; Trx1, thioredoxin 1.
GSR is an enzyme that catalyzes the reduction of GSSG to GSH, which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell. The present study demonstrated that GSR immediately underwent oxidation in response to H2O2 and returned to its reduced form 1 h following H2O2 treatment (Fig. 3E and F). Redox western blotting revealed that GSR displayed a high molecular weight band (~150 kDa) following stimulation with H2O2 using the NEM derivatization method (Fig. 3G, upper panel), which disappeared under reducing conditions (Fig. 3E, lower panel). NAC pretreatment significantly shifted GSR levels from the oxidized to the reduced form (Fig. 3G, upper panel and Fig. 3H). The redox states of Trx, Prx and GSR were modified by NAC pretreatment during oxidative stress, thus indicating that the cytoprotective function of NAC is associated with regulation of the redox state of intracellular antioxidants.
NAC pretreatment decreases H2O2-induced phosphorylation of ASK1
ASK1, which is a serine/threonine protein kinase, is a ROS-sensitive mitogen-activated protein kinase (MAPK) kinase kinase and a key mediator in cell death. The active form of ASK1 (p-ASK1) functions as a signaling node for several MAPK kinases, resulting in activation of p38MAPK and c-Jun N-terminal kinase (JNK), and regulation of cell survival and death (14). Trx1 has been reported to directly bind to the N-terminal regulatory domain of ASK1, resulting in inhibition of apoptosis. The interaction between Trx1 and ASK1 is highly dependent on the redox status of Trx. Reduced Trx1 interacts with and inhibits ASK1 activity, whereas oxidized Trx1 has decreased affinity for ASK1 (15). The present study revealed that NAC pretreatment increased the levels of reduced Trx1 under oxidative conditions; therefore, this study investigated whether NAC pretreatment influences H2O2-induced phosphorylation of ASK1 in H9c2 cells. As shown in Fig. 3I, phosphorylation of ASK1 was induced following treatment with H2O2 for 15 min. Conversely, NAC pretreatment alleviated activation of ASK1 (Fig. 3J), which is consistent with the increased levels of reduced Trx1 (Fig. 3A).
NAC pretreatment decreases H2O2-induced oxidation of PTEN and increases H2O2-induced phosphorylation of AKT
The tumor suppressor PTEN is a phosphatase that dephosphorylates phosphatidylinositol (3,4,5)-trisphosphate, which is the lipid product of the class I PI3Ks, and suppresses growth and proliferation of several cell types (16). The phosphatase activity of PTEN is redox-dependent and is inhibited by H2O2-induced transient oxidation of PTEN, which subsequently activates PI3K-dependent signaling, including AKT phosphorylation, and promotes cell survival (17). Cao et al and Schwertassek et al reported that Trx1 and Prx1 increase the reduced form of PTEN by protecting it from oxidation-induced inactivation (18,19). The present study demonstrated that NAC pretreatment protected Trx1 and Prx1 from H2O2-induced oxidation. In the present study, the redox state of PTEN and phosphorylation of AKT were investigated. The present results demonstrated that PTEN underwent oxidation following H2O2 stimulation in H9c2 cells, and the reduced form was recovered ~2 h after H2O2 treatment (Fig. 4A and B). Notably, the levels of H2O2-induced oxidized PTEN were decreased by NAC pretreatment, which corresponded with an increase in the reduced form (Fig. 4C and D). Unexpectedly, phosphorylation of AKT was significantly increased by NAC pretreatment (Fig. 4E and F).
Discussion
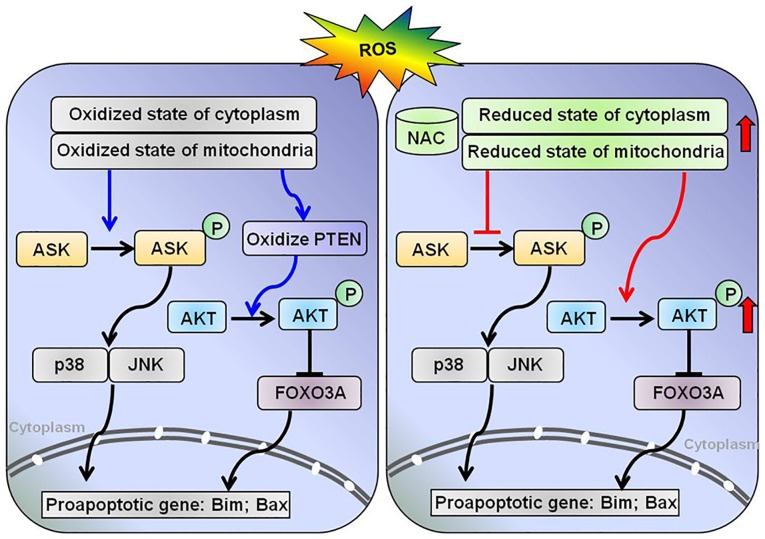
The antioxidant activity of NAC results from its free radical-scavenging properties, which occur either directly by modulating the redox potentials of thiols, or by increasing cellular levels of GSH. The present study revealed that NAC pretreatment protected H9c2 cells from H2O2-induced apoptosis by modifying the intracellular redox state and modulating downstream redox signaling pathways, including the proapoptotic kinase ASK1 and the prosurvival kinase AKT (Fig. 5).
Figure 5.
Schematic diagram of the working hypothesis of NAC-associated signaling in H9c2 cells during oxidative stress. It was hypothesized that NAC maintains the reduced state in the cytoplasm and promotes antioxidant pathways.
Oxidative stress is caused by an imbalance between reduced and oxidized biomolecules within cells, in favor of the latter, thus resulting in alterations that are deleterious to vital cell functions (20). As a consequence, numerous cellular compounds undergo redox modifications, some of which function in signaling transduction. Numerous key regulators of redox signaling are members of the Trx family, including Trx1 and Prx1. Trx1 and Prx1 are characterized by their active site motifs that contain one or two cysteine residues (2). These thiol groups are essential for the reduction of protein disulfide bonds, deglutathionylation and denitrosylation. During these processes, the active sites of Trx form a protein disulfide bond. Usually, oxidation of Trx is reduced by Trx reductase, which uses NADPH as a cofactor (21). The present study demonstrated that repletion of GSH by NAC attenuated the oxidation of Trx1 under oxidative conditions in H9c2 cells. Szadkowski and Myers also reported that pretreatment of endothelial cells with NAC resulted in partial protection of Trx1 from oxidation by acrolein, a reactive aldehyde (22). The underlying mechanism is possibly due to decreased formation of protein disulfides in the active sites of Trx1 or increased reduction of oxidized Trx1 by Trx reductase.
ASK1 is activated by ROS via Trx1 and/or Grx1. Reduced Trx1 and Grx1 bind to the N- and C-terminal domains of ASK1, respectively, thereby inhibiting its activation. Stress-induced oxidation of Trx1 or Grx1 leads to their dissociation from the complex and subsequent activation of the kinase (15,23,24). In addition, binding of Trx1 to ASK1 targets the kinase for degradation via the ubiquitin pathway (25). Notably, this study demonstrated that NAC pretreatment attenuated H2O2-induced oxidation of Trx1 and Prx1, and decreased phosphorylation of ASK1, which may subsequently result in a reduction in caspase-3, -8 and -9 activities. This finding may be associated with inhibition of the p38/JNK signaling pathway.
The GSH/GPx/Grx signaling cascade is another important antioxidant pathway (26). GPx and Grx use reduced GSH to reduce hydrogen/lipid peroxide or oxidation products; during these processes, reduced GSH is oxidized to GSSG; GSSG can be reduced back to GSH by GSR and NADPH. Oxidation of GSR may contribute to the increased levels of GSSG and the decreased GSH/GSSG ratio induced by H2O2. The present study revealed that the oxidized form of GSR was decreased in the NAC pretreatment group, which indicated that redox regulation of GSR may be important to intracellular redox status.
The oxidation of PTEN induced by H2O2 activates the AKT signaling pathway and protects cells from oxidative stress-induced cell death. In the present study, pretreatment with NAC markedly attenuated PTEN oxidation induced by H2O2, which was expected to decrease the phosphorylation of AKT. However, the expression levels of p-AKT were increased by NAC pretreatment. The phosphorylation of AKT is necessary for its anti-apoptotic function and cell survival signal transduction in response to oxidative stress. These data indicated that the phosphorylation of AKT under oxidative conditions is regulated by both oxidation of PTEN and other factors. Murata H et al reported that, under oxidative stress, AKT is transiently phosphorylated and undergoes disulfide bond formation between Cys-297 and Cys-311; dephosphorylation corresponds with an increased association with protein phosphatase 2A in H9c2 cells. In addition, Grx may protect AKT from H2O2-induced oxidation and maintain phosphorylation of AKT, thus inhibiting apoptosis (27). The redox status of Grx was difficult to detect in the present study, due to the poor quality of the Grx antibody and the lack of an appropriate redox blot protocol for Grx. The AKT signaling pathway serves a pivotal role in inhibiting apoptosis through activation of downstream effector molecules. Transcription factor forkhead box O3 (FoxO3a) is one of the most important downstream targets of AKT signaling and a crucial regulator of pro-apoptotic genes, such as Bcl-2-like protein 11 (28,29). It has been reported that inhibition of AKT promotes FOXO3a-dependent apoptosis in several cell types (30). Based on these findings, it may be hypothesized that NAC inhibits H2O2-induced apoptosis via the AKT/FOXO3a signaling pathway (Fig. 5).
Physiological, homeostatic and intracellular ROS are maintained at low levels by various enzyme systems. ROS are becoming increasingly appreciated as second messengers and signaling molecules that regulate various physiological responses (31). At excessive concentrations, H2O2 can cause biological damage. Conversely, it also promotes oxidation-induced inactivation of phosphatases, such as PTEN, thus mediating cell survival signal transduction. Oxidative stress is originally defined as an imbalance between the production of oxidants or ROS, and their elimination by antioxidants. The redefinition of ‘oxidative stress’ is based on alterations in observable post-translational protein thiol modifications that are central to redox regulation and control (26). Redox western blot analysis and redox-sensitive GFPs provide means to quantify thiol/disulfide redox alterations in specific subcellular compartments (26). It is important to analyze the redox modification of protein thiols to understand the role of redox modifications on protein function.
In conclusion, to the best of our knowledge, the present study is the first to reveal that NAC pretreatment alleviates oxidation of intracellular antioxidant proteins, in order to inhibit oxidative stress-induced cardiomyocyte apoptosis. These findings may broaden the clinical applications of NAC in ROS-asssociated diseases, such as myocardial infarction and Alzheimer’s disease.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81270279 and 81471897) and the Hunan Natural Science Foundation (grant no. 2013JJ1009).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
HZ conceived the study and designed the experiments. XL, LW and JC performed the experiments. KL, ML and HW collected and analyzed the experimental results. XL drafted and revised the article. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH. Thioredoxins, glutaredoxins, and peroxire-doxins-molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 2013;19:1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmgren A, Johansson C, Berndt C, Lönn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutare-doxin systems. Biochem Soc Trans. 2005;33:1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- 4.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 6.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 18302013:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Mayer M, Noble M. N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sci USA. 1994;91:7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng YW, Buller CL, Charpie JR. Impact of N-acetylcysteine on neonatal cardiomyocyte ischemia-reperfusion injury. Pediatr Res. 2011;70:61–66. doi: 10.1203/PDR.0b013e31821b1a92. [DOI] [PubMed] [Google Scholar]
- 9.Duan JL, Wang JW, Guan Y, Yin Y, Wei G, Cui J, Zhou D, Zhu YR, Quan W, Xi MM, Wen AD. Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacol Sin. 2013;34:487–495. doi: 10.1038/aps.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Mao X, Li H, Qiao S, Xu A, Wang J, Lei S, Liu Z, Ng KF, Wong GT, et al. N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic Biol Med. 2013;63:291–303. doi: 10.1016/j.freeradbiomed.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Sitasawad SL. N-acetylcysteine prevents glucose/glucose oxidase-induced oxidative stress, mitochondrial damage and apoptosis in H9c2 cells. Life Sci. 2009;84:328–336. doi: 10.1016/j.lfs.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Poynton RA, Hampton MB. Peroxiredoxins as biomarkers of oxidative stress. Biochim Biophys Acta. 18402014:906–912. doi: 10.1016/j.bbagen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Limphong P, Pieper J, Liu Q, Rodesch CK, Christians E, Benjamin IJ. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012;26:1442–1451. doi: 10.1096/fj.11-199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishida K, Otsu K. The role of apoptosis signal-regulating kinase 1 in cardiomyocyte apoptosis. Antioxid Redox Signal. 2006;8:1729–1736. doi: 10.1089/ars.2006.8.1729. [DOI] [PubMed] [Google Scholar]
- 15.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK)1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leslie NR, Kriplani N, Hermida MA, Alvarez-Garcia V, Wise HM. The PTEN protein: Cellular localization and post-translational regulation. Biochem Soc Trans. 2016;44:273–278. doi: 10.1042/BST20150224. [DOI] [PubMed] [Google Scholar]
- 17.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, Manevich Y, Beeson C, Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwertassek U, Haque A, Krishnan N, Greiner R, Weingarten L, Dick TP, Tonks NK. Reactivation of oxidized PTP1B and PTEN by thioredoxin 1. FEBS J. 2014;281:3545–3558. doi: 10.1111/febs.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris C, Hansen JM. Oxidative stress, thiols, and redox profiles. Methods Mol Biol. 2012;889:325–346. doi: 10.1007/978-1-61779-867-2_21. [DOI] [PubMed] [Google Scholar]
- 21.Whayne TF, Jr, Parinandi N, Maulik N. Thioredoxins in cardiovascular disease. Can J Physiol Pharmacol. 2015;93:903–911. doi: 10.1139/cjpp-2015-0105. [DOI] [PubMed] [Google Scholar]
- 22.Szadkowski A, Myers CR. Acrolein oxidizes the cytosolic and mitochondrial thioredoxins in human endothelial cells. Toxicol. 2008;243:164–176. doi: 10.1016/j.tox.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song JJ, Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003;373:845–853. doi: 10.1042/bj20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–46575. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.RES.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 26.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 27.Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K, Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- 28.Wu D, Liang M, Dang H, Fang F, Xu F, Liu C. Hydrogen protects against hyperoxia-induced apoptosis in type II alveolar epithelial cells via activation of PI3K/Akt/Foxo3a signaling pathway. Biochem Biophys Res Commun. 2018;495:1620–1627. doi: 10.1016/j.bbrc.2017.11.193. [DOI] [PubMed] [Google Scholar]
- 29.Wang YQ, Cao Q, Wang F, Huang LY, Sang TT, Liu F, Chen SY. SIRT1 protects against oxidative stress induced endothelial progenitor cells apoptosis by inhibiting FOXO3a via FOXO3a ubiquitination and degradation. J Cell Physiol. 2015;230:2098–2107. doi: 10.1002/jcp.24938. [DOI] [PubMed] [Google Scholar]
- 30.Das TP, Suman S, Alatassi H, Ankem MK, Damodaran C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016;7:e2111. doi: 10.1038/cddis.2015.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.