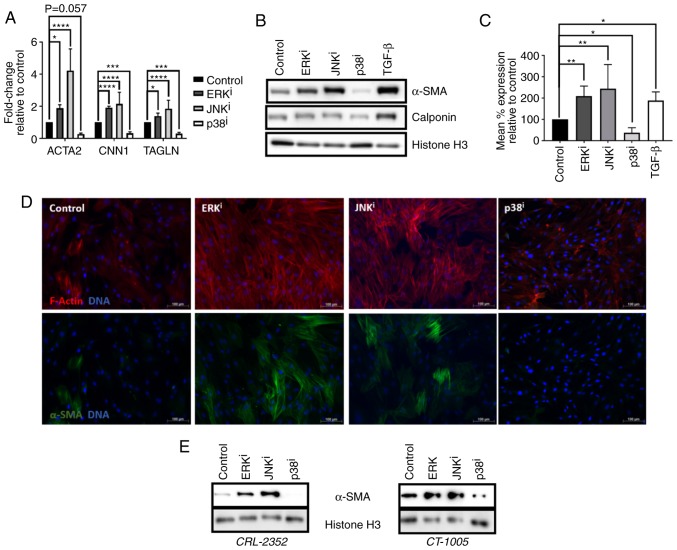
Figure 1.
Effects of MAPK inhibitors on fibroblast activation. (A–D) CRL-2097 human dermal fibroblasts were cultured under control conditions, or in the presence of 10 µM U0126 (ERKi), 10 µM SP600125 (JNKi), 10 µM SB202190 (p38i) or 10 ng/ml exogenous recombinant human TGF-β1, and harvested at day 4 in culture. (A) Expression of myofibroblast marker transcripts ACTA2, CNN1 and TAGLN were determined by reverse transcription-quantitative polymerase chain reaction, normalized to the expression of GAPDH, and expressed as fold change relative to the expression of control samples. There were 4 biological replicates/condition. Error bars represent standard deviation. Statistics were performed by one-way ANOVA with multiple comparisons using the Holm-Sidak post-hoc test. *P<0.05, ***P<0.001 and ****P<0.0001. (B) Western blot analysis was performed on myofibroblast markers α-SMA and calponin on whole cell lysates. Histone H3 was used as a loading control. (C) Fibroblasts were fixed and subjected to indirect flow cytometry, and the expression of α-SMA was compared with the expression in control fibroblasts. There were 4 biological replicates/condition. Statistics were performed by one-way ANOVA with multiple comparisons using the Holm-Sidak post-hoc test. *P<0.05 and **P<0.01. (D) Fibroblasts were fixed and stained by immunofluorescence for α-SMA, and F-actin was visualized using fluorescently-labeled phalloidin. Nuclei were counterstained with Hoechst. Scale bars, 100 µm. (E) CRL-2352 and CT-1005 fibroblasts were cultured under control conditions or in the presence of a MAPK inhibitor, and harvested at day 4 in culture. Western blot analysis was performed for detection of α-SMA. Histone H3 was used as a loading control. MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; ANOVA, analysis of variance; ACTA2, α-SMA; α-SMA, smooth muscle α actin; CNN1, calponin 1; TAGLN, transgelin; TGF-β, transforming growth factor-β; i, inhibitor.