Abstract
Introduction
The objective of this study was to determine the frequency and clinical and cognitive characteristics of preclinical Alzheimer's disease (AD) in a Japanese population to effectively design and conduct future preventive trials on preclinical AD.
Methods
Three-year longitudinal data from cognitively normal participants who underwent cerebrospinal fluid biomarker measurement and/or amyloid positron emission tomography in the Japanese Alzheimer's Disease Neuroimaging Initiative, were analyzed. Comparisons between participants with and without amyloid β (Aβ) accumulation, and between those with and without elevated tau levels tau among participants with Aβ accumulation were performed.
Results
Among 84 participants with available cerebrospinal fluid biomarker and/or amyloid positron emission tomography data, 19 (22.6%) exhibited Aβ accumulation. The frequency of APOE ε4 alleles was significantly higher in participants with Aβ accumulation. There were no significant differences in any of the cognitive tests at the baseline; however, participants with Aβ accumulation exhibited a decline in clock drawing test (linear mixed-effects model, P = .008) and a tendency toward loss of practice effects in the Mini-Mental State Examination and the logical memory over time. Although it did not reach statistical significance, the analysis indicated a decline in measurements of executive function over time in participants with elevated tau levels compared with those with normal tau levels.
Discussion
The frequency of preclinical AD in the Japanese Alzheimer's Disease Neuroimaging Initiative was lower than in similar studies because of the younger age of the participants and lower frequency of APOE ε4 carriage. Although limitations in sample size precluded definitive conclusions, the results suggest that even in the preclinical phase of AD, loss of practice effects in episodic memory tests and at a later stage, decline in executive function, are present. These findings may be useful for recruitment of individuals with preclinical AD and establishing a novel cognitive composite for use in clinical trials on preclinical AD.
Keywords: J-ADNI, Preclinical Alzheimer's disease, APOE, Practice effect, Episodic memory, Executive function
1. Introduction
Given the recent consecutive failures in clinical trials of disease modifying therapies for Alzheimer's disease (AD), there has been growing interest in the asymptomatic phase of AD (i.e., “preclinical AD”) as a therapeutic target of preventive intervention. During the preclinical AD phase, only pathophysiological changes of AD can be detected using biomarkers. Cognitive impairment, however, is virtually undetectable using conventional cognitive tests according to definition of preclinical AD. Therefore, to effectively design and successfully conduct clinical trials on preclinical AD, clinical and cognitive characteristics of preclinical AD in addition to biomarker profiles should be elucidated.
To date, a few previous studies have investigated longitudinal changes in clinical and cognitive profiles in cognitively normal (CN) elderly individuals with amyloid β (Aβ) accumulation proven by biomarker data. Donohue et al. reported that CN individuals with Aβ accumulation exhibited a tendency toward worse scores in the Clinical Dementia Rating (CDR) sum of boxes and the Mini-Mental State Examination (MMSE) at 4 years of follow-up [1]. On the other hand, the INSIGHT-preAD did not reveal any difference in scores of the MMSE and the CDR at 30 months of follow-up [2].
The Japanese Alzheimer's Disease Neuroimaging Initiative (J-ADNI) was the first large-scale longitudinal observational study in Japan, conducted between 2008 and 2014, following the North American ADNI (NA-ADNI), to elucidate the natural history of the early stages of AD in the Japanese population. We recently reported that individuals with mild cognitive impairment or mild AD exhibited similar profiles of cognitive and functional decline in the J-ADNI and the NA-ADNI [3]. The J-ADNI also included a 3-year follow-up with intensive assessments of 154 CN individuals from a total of 537 participants between 60 and 84 years of age. In the present study, we investigated the frequency of preclinical AD in the Japanese population and performed analyses and comparisons of clinical and cognitive data from participants with and without Aβ accumulation, to characterize demographics and longitudinal cognitive and functional decline in Japanese individuals with preclinical AD. This information is advantageous for the effective recruitment of individuals with preclinical AD and in developing cognitive outcome measures in clinical trials on preclinical AD.
2. Methods
2.1. Acquisition of the J-ADNI data and ethics approval
The entire data set of the J-ADNI was downloaded from the National Bioscience Database Center (Tokyo, Japan, https://humandbs.biosciencedbc.jp/en/hum0043-v1) on October 12, 2017, on approval of the Ethics Committees of the University of Tokyo Graduate School of Medicine (Tokyo, Japan; approval number 11628) and the National Bioscience Database Center (DU-0039).
2.2. Participants and variables analyzed
The study protocol used in the J-ADNI has been described in detail elsewhere [3]. To describe the eligibility criteria briefly, CN participants in the J-ADNI were required to be 60 to 84 years of age, and have a physician's diagnosis of not having dementia or mild cognitive impairment, a CDR score of 0, and an MMSE score of 24 to 30 at the time of screening. Only participants with results of amyloid positron emission tomography (PET) and/or cerebrospinal fluid (CSF) biomarker at the baseline assessment were included in the analyses. Positive Aβ accumulation was defined as either positive or equivocal amyloid deposition on 11C-PiB or 11C-BF–227 PET by visual reads [4], or CSF Aβ1–42 below 333 pg/mL at the baseline assessment [3]. Thus, negative Aβ accumulation was defined as either negative scan on amyloid PET or CSF Aβ1–42 > 333 pg/mL. Elevated tau level was defined as CSF phosphorylated tau (p-tau) > 45 pg/mL at the baseline assessment. Brain tau status could be defined only among participants who underwent CSF biomarker analyses at the baseline. These cutoff values for CSF biomarkers were established using receiver operating characteristic curve analysis of CSF Aβ1–42 and p-tau measurements at the baseline from 35 AD and 53 CN cases.
Demographic data, including age, sex, education, family history within first-degree relatives and frequency of APOE ε4 alleles, were analyzed. For clinical scales, the CDR, the Neuropsychiatric Inventory Questionnaire, the Functional Assessment Questionnaire, and the Geriatric Depression Scale were analyzed. Cognitive measures were selected to assess each cognitive domain and global cognition: the MMSE and the Alzheimer's Disease Assessment Scale–cognitive subscale 13 for global cognition; the logical memory from the Wechsler Memory Scale-Revised for episodic memory; the digit span and the digit symbol substitution test from the Wechsler Adult Intelligence Scale-Revised, category fluency, and trail making test for executive function; the Boston naming test for semantic function; and the clock drawing test and clock copying test for visuospatial function.
2.3. Statistical analysis
All the statistical analyses were performed using JMP Pro 14 (SAS Institute Inc., Cary, NC, USA). For continuous data, comparisons between two groups were performed using the Student's t-test; the Mann-Whitney U test was used when an outlier(s) existed. Group comparisons for categorical measures, such as sex and presence of family history of dementia, were performed using the chi-squared test or the Fisher's exact test, as appropriate. Longitudinal changes in clinical scales and cognitive tests were compared using linear mixed-effects models. The models were controlled for age at the baseline and APOE ε4 carriage, which are known to be associated with cognition, sex, years of education, and their interaction with time. Individuals were included as a random effect in the models. All P values were generated for the interactive effect between group and time. Differences with P < .05 were considered to be statistically significant.
3. Results
3.1. Frequency of Aβ-positive individuals among the CN elderly in the J-ADNI cohort
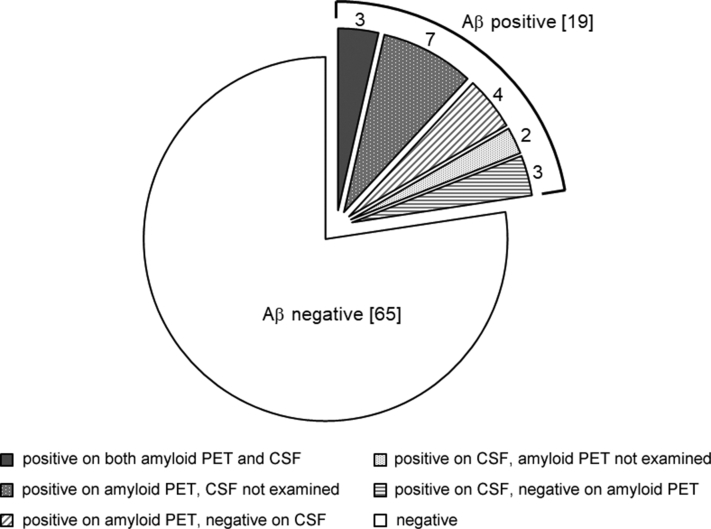
Of 154 CN individuals, 84 participants underwent either amyloid PET or lumber puncture at the baseline assessment, and 19 (22.6%) and 65 (77.4%) participants were defined as positive Aβ accumulation and negative Aβ accumulation, respectively (Fig. 1). Among the participants with Aβ accumulation, seven demonstrated disagreement in amyloid accumulation status between amyloid PET and CSF: four demonstrated positive Aβ accumulation on 11C-PiB PET and negative Aβ accumulation on CSF; one exhibited positive Aβ accumulation on CSF and negative Aβ accumulation on 11C-PiB PET; and two demonstrated positive Aβ accumulation on CSF and negative Aβ accumulation on 11C-BF–227 PET. Similar disagreements between Aβ biomarkers were previously reported in the NA-ADNI and another study [5], [6].
Fig. 1.
Breakdown of cognitively normal participants in whom the Aβ biomarker was examined. Numbers in brackets indicate the number of participants in each category. Abbreviations: Aβ, amyloid β; PET, positron emission tomography; CSF, cerebrospinal fluid.
3.2. Comparison of clinical and cognitive characteristics between participants with and without Aβ accumulation
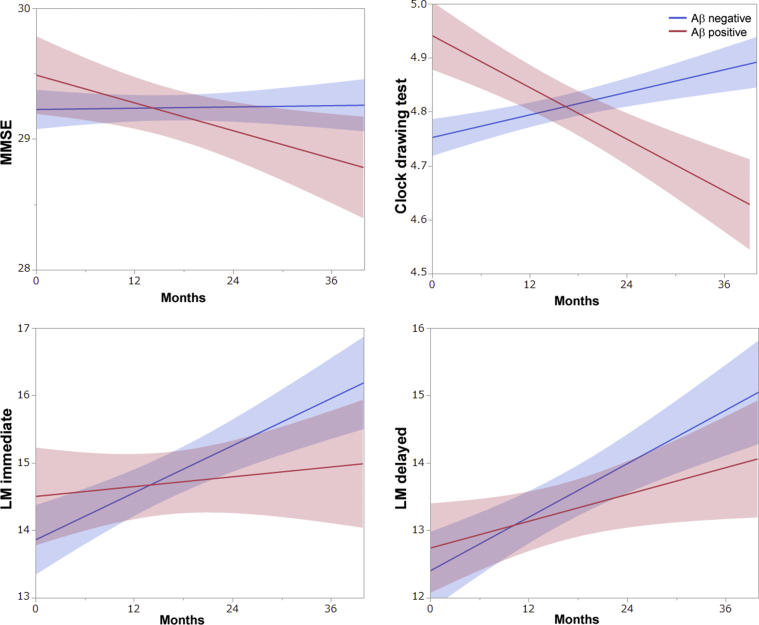
The mean age at the baseline (68.4 years for Aβ positive, 67.8 years for Aβ negative, Student's t-test, P = .50), sex, education, and family history were not statistically different between the groups (Table 1). Participants with Aβ accumulation carried greater frequencies of APOE ε4 alleles (Fisher's exact test, P = .003). There were no significant differences in clinical scales and psychometric tests at the baseline. CSF p-tau levels were significantly higher in participants with Aβ accumulation (48.8 pg/mL in Aβ positive, 35.2 pg/mL in Aβ negative, Student's t-test, P = .002). No participants in the analysis converted from CN to mild cognitive impairment or dementia within the 3-year follow-up. Comparison of clinical scales and cognitive tests at the baseline assessment between participants with and without Aβ accumulation revealed no significant differences, except for the Geriatric Depression Scale and the clock drawing test (Table 2). The participants without Aβ accumulation exhibited a tendency toward a higher—but not clinically meaningful—score on the Geriatric Depression Scale (0.63 for Aβ positive, 1.45 for Aβ negative, Mann-Whitney U test, P = .002). In terms of longitudinal changes, linear mixed-effects models revealed significant differences only in the clock drawing test (0.20/year for Aβ positive, −0.038/year for Aβ negative, P = .008) (Fig. 2). Although differences in other scores did not reach the threshold for statistical significance, participants without Aβ accumulation tended to remain psychiatrically stable, and exhibited improvement in MMSE scores and greater improvement in scores of the logical memory immediate recall and delayed recall with time (Fig. 2), which may have represented practice effects due to repetitive testing.
Table 1.
Demographics and baseline CSF biomarkers of the study participants
| Baseline characteristics | Aβ+ | Aβ− | P value |
|---|---|---|---|
| N | 19 | 65 | |
| Age, mean (SD), years | 68.6 (4.5) | 67.6 (5.4) | .5 |
| Sex, female % | 52.6 | 46.2 | .62 |
| Education, mean (SD), years | 13.4 (1.6) | 14 (2.6) | .36 |
| Family history of dementia, % | 63.2 | 43 | .12 |
| APOE ε4 alleles, N | .003 | ||
| 0 | 9 | 54 | |
| 1 | 9 | 10 | |
| 2 | 1 | 1 | |
| CSF Aβ42, mean (SD), pg/mL | 301.8 (98.2) | 515.2 (17.1) | <.0001 |
| CSF p-tau, mean (SD), pg/mL | 48.8 (3.8) | 35.2 (2.0) | .002 |
Abbreviations: Aβ+, participants with Aβ accumulation; Aβ−, participants without Aβ accumulation; SD, standard deviation; CSF, cerebrospinal fluid; Aβ, amyloid β; p-tau, phosphorylated tau.
NOTE. P values were determined by use of the Student's t-test for age, education, and CSF biomarkers, the chi-squared test for sex and family history, and the Fisher's exact test for the frequency of APOE ε4 alleles.
Table 2.
Clinical scales and cognitive tests at the baseline assessment of the study participants
| Baseline clinical scores and cognitive tests | Aβ+ | Aβ- | P value |
|---|---|---|---|
| CDR-SB | 0 | 0.085 | .06 |
| NPI-Q | 0.11 | 0.14 | .72 |
| FAQ | 0.21 | 0.08 | .05 |
| GDS | 0.63 | 1.45 | .02 |
| MMSE | 29.3 | 29.2 | .86 |
| ADAS-cog 13 | 8.83 | 7.4 | .18 |
| Logical memory, immediate | 14.4 | 13.7 | .39 |
| Logical memory, delayed | 12.6 | 12.3 | .78 |
| Digit span forward | 9.17 | 9 | .5 |
| Digit span backward | 6.11 | 6.73 | .18 |
| Category fluency, animal | 20.1 | 19.5 | .68 |
| Category fluency, vegetable | 15.9 | 16.3 | .57 |
| Trail making test -A | 40.5 | 36.9 | .39 |
| Trail making test -B | 94.6 | 97.6 | .82 |
| Digit symbol substitution test | 63.8 | 66.7 | .28 |
| Boston naming test | 28.8 | 29 | .71 |
| Clock drawing test | 5 | 4.7 | .04 |
| Clock copying test | 4.95 | 4.97 | .69 |
Abbreviations: Aβ, amyloid β; Aβ+, participants with Aβ accumulation; Aβ−, participants without Aβ accumulation; CDR-SB, clinical dementia rating sum of boxes; NPI-Q, Neuropsychiatric Inventory Questionnaire; FAQ, Functional Assessment Questionnaire; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; ADAS-cog 13, Alzheimer's Disease Assessment Scale–cognitive subscale 13.
NOTE. All P values were determined by use of the Mann-Whitney U test.
Fig. 2.
Comparison of longitudinal changes in cognitive tests between participants with and without Aβ accumulation. Each plot shows the regression line with 95% confidence interval. Abbreviations: Aβ, amyloid β; MMSE, Mini-Mental State Examination; LM immediate, logical memory immediate recall; LM delayed, logical memory delayed recall.
3.3. Comparison between participants with elevated and normal tau levels in those with preclinical AD
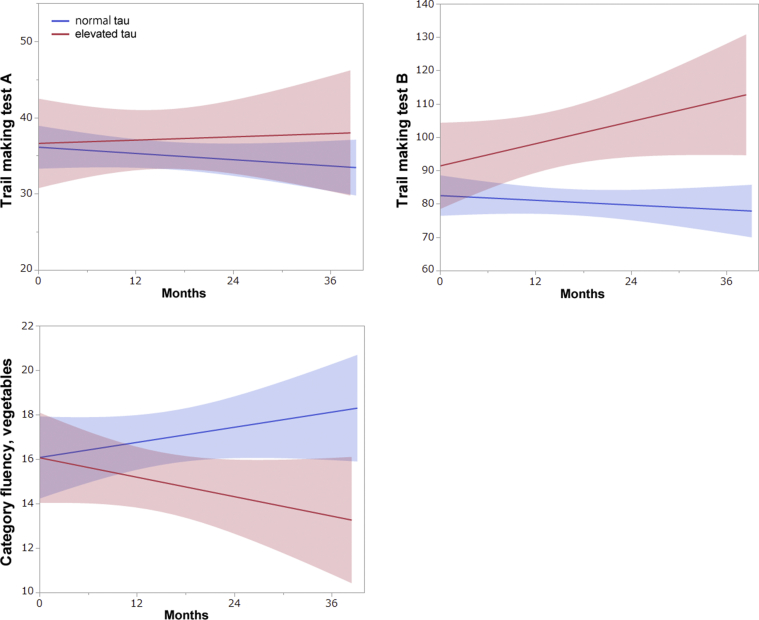
Among 19 participants with preclinical AD, 12 had available CSF p-tau data at the baseline: five participants with elevated tau levels; and seven with normal tau levels. There were no differences in the mean age at the baseline (67.7 years for those with elevated tau, 67.9 years for those with normal tau levels, Student's t-test, P = .97), and other demographic data between the groups. Mean CSF p-tau was 71.6 pg/mL in those with elevated tau and 32.6 pg/mL in those with normal tau levels (Student's t-test, P = .0018). There was no statistically significant difference in any of the clinical scales and cognitive tests at the baseline assessment; however, participants with elevated tau levels demonstrated a trend toward lower scores on the logical memory (immediate recall: 13.4 in elevated tau and 15.4 in normal tau, P = .37; delayed recall: 11.2 in elevated tau and 13.7 in normal tau, P = .32), and the digit symbol substitution test (61.8 in elevated tau and 66.4 in normal tau, P = .33) with limitations in sample size. With regard to longitudinal changes, there was a significant difference only in the completion time of the trail making test A (0.43/year for elevated tau, −0.75/year for normal tau, P = .02) (Fig. 3). Moreover, there was a trend in which participants with elevated tau levels became progressively worse, whereas participants with normal tau levels were improving in the category fluency to vegetables, although it did not reach statistical significance (linear mixed-effects model, P = .28). All of these tests are considered to be measures of executive function.
Fig. 3.
Comparison of longitudinal changes in cognitive tests between participants with elevated and normal tau levels among those with preclinical Alzheimer's disease. Each plot shows the regression line with 95% confidence interval.
4. Discussion
This was the first study in which the prevalence of individuals with preclinical AD, and clinical and cognitive profiles of those with preclinical AD, was investigated in a Japanese population. The Aβ positivity rate among CN elderly individuals was lower in the J-ADNI than in similar large-scale studies: 38.1% of participants 65 to 85 years of age in the NA-ADNI [7]; 33% of participants ≥60 years of age in the Australian Imaging, Biomarkers and Lifestyle [8]; and 28% of participants 70 to 85 years of age in the INSIGHT-preAD [2]. Carriage of APOE ε4 allele is known to be a major risk factor, not only for AD dementia [9], but also for preclinical Aβ accumulation [10]; however, it did not fully account for the Aβ positivity rate being lower in our study. The frequency of APOE ε4 among all participants in the analysis was 25%, which is lower than in the Australian Imaging, Biomarkers and Lifestyle (43%), but is essentially comparable with the figures reported in the NA-ADNI (28%) and the INSIGHT-preAD (20%). The major reason for the lower Aβ positivity rate in the J-ADNI was believed the younger age of the cohort, given that age is known to be another major risk factor for Aβ accumulation independent of APOE ε4 [8], [10]. The frequency of APOE ε4 carriers among CN individuals was higher in the J-ADNI than in a population-based study by the Japanese Genetic Study Consortium for AD (17.5%) [11]. Although no precedent longitudinal cohort studies investigating early stage of AD were conducted before the J-ADNI in Japan, and the participants were recruited solely from the community, not from other cohort studies in which participants or their family members had participated, study participation itself may have contributed to enrichment of APOE ε4 carriers to some degree.
Similar to reports from several other previous studies [1], [2], [10], CN participants with Aβ accumulation in the J-ADNI tended to exhibit a significantly greater number of APOE ε4 alleles than those without Aβ accumulation [1], [2]. Despite repetition of cognitive tests during the follow-up period, the participants with Aβ accumulation exhibited a trend toward loss of practice effects in the MMSE and the logical memory. A previous study reported that individuals who maintained a CDR score of 0 for 3 years exhibited practice effects on episodic memory measures [12]. Although Aβ biomarkers were not included in the previous study, as cognitively stable individuals are considered to have a lower possibility of Aβ accumulation, previous results match with those in our study. Thus, detection of APOE ε4 carriage and loss of practice effects in repetition of memory tests may be useful to effectively enrich individuals with preclinical AD before measurement of an Aβ biomarker.
We next probed which cognitive domain is more impaired in individuals with preclinical AD. Donohue et al. developed the preclinical Alzheimer cognitive composite (PACC) to measure amyloid-related cognitive decline in preclinical AD for use in a clinical trial on preclinical AD. The PACC is composed of the MMSE, two memory tests: the logical memory and the free and cued selective reminding test, and the digit symbol substitution test [7]. Individuals with preclinical AD exhibited longitudinal decline on every component of the PACC [13]. Furthermore, Papp et al. developed the PACC5, which added category fluency tests to the PACC as a cognitive composite more sensitive to amyloid-related cognitive decline in preclinical AD than the PACC [14]. Monsell et al. reported a greater rate of decline in the attention and working memory domain in individuals with preclinical AD than CN individuals without AD pathology [15]. These studies suggest a decline, not only in episodic memory but also in executive function, can be seen in preclinical AD. Although there was a limitation in sample size in our study, we further divided the participants with Aβ accumulation into those with elevated and normal tau levels. Each group corresponded to stage 1 and stage 2/3 of preclinical AD, respectively [16]. Our results suggested that individuals in stage 2/3 of preclinical AD may already present measurable—albeit not clinically significant—decline in episodic memory, and begin declining in multiple measures of executive function. This finding will help inform the development of a novel cognitive composite to assess cognitive decline in preclinical AD. CSF p-tau is known to be correlated with neocortical neurofibrillary tangle pathology [17]; therefore, it appears reasonable that decline in episodic memory was seen first, followed by the decline in executive function, which reflects the spatial progression of neurofibrillary tangle pathology as the disease progresses [18].
In conclusion, this study demonstrated that preclinical AD in Japanese individuals did not differ from that reported in other studies from western countries. Characteristics of cognitive profiles of preclinical AD may lie in the decline in executive function as well as episodic memory, becoming more obvious as the stage progresses. To draw definitive conclusions, studies with larger sample sizes and longer follow-up periods on preclinical AD are needed. To support ongoing global clinical trials on preclinical AD, multimodal comparison of participants with preclinical AD between the J-ADNI and the NA-ADNI is necessary in the near future.
Research in Context.
-
1.
Systematic review: The Pubmed database was searched to identify large-scale longitudinal studies examining the frequency of preclinical Alzheimer's disease (AD) and cognitive profiles of individuals with preclinical AD. The present study was the first study to examine these in a Japanese population using data from the Japanese Alzheimer's Disease Neuroimaging Initiative.
-
2.
Interpretation: Analyses revealed that the frequency of preclinical AD was lower in the Japanese Alzheimer's Disease Neuroimaging Initiative cohort because of younger age and lower frequency of APOE ε4 carriage. Individuals with amyloid β accumulation tended to lack practice effects in the Mini-Mental State Examination and the logical memory, and score poorly on executive function tests. These cognitive profiles of preclinical AD were consistent with results from previous studies.
-
3.
Future directions: Results from the present study may inform the development of more sensitive cognitive composites for future prevention trials and the consideration of repetitive memory tests as prescreening to effectively recruit individuals with preclinical AD.
Acknowledgments
The authors thank especially the participants and staff who contributed to the J-ADNI. The data used for this research were originally obtained by the J-ADNI research project/group led by Principal Investigator Takeshi Iwatsubo, MD, and are available at the website of the National Bioscience Database Center (NBDC)/the Japan Science and Technology Agency (JST).
This work was supported by the Japan Agency for Medical Research and Development [grant numbers 18DK0207028, 18DK0207027] and the JST [grant number 17K09794]. The J-ADNI was supported by the following grants: Translational Research Promotion Project from the New Energy and Industrial Technology Development Organization of Japan; Research on Dementia, Health Labor Sciences Research Grant; Life Science Database Integration Project of Japan Science and Technology Agency; Research Association of Biotechnology (contributed by Astellas Pharma Inc., Bristol-Myers Squibb, Daiichi-Sankyo, Eisai, Eli Lilly and Company, Merck-Banyu, Mitsubishi Tanabe Pharma, Pfizer Inc., Shionogi & Co., Ltd., Sumitomo Dainippon, and Takeda Pharmaceutical Company), Japan, and a grant from an anonymous Foundation.
References
- 1.Donohue M.C., Sperling R.A., Petersen R., Sun C.K., Weiner M.W., Aisen P.S. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA. 2017;317:2305–2316. doi: 10.1001/jama.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois B., Epelbaum S., Nyasse F., Bakardjian H., Gagliardi G., Uspenskaya O. Cognitive and neuroimaging features and brain β-amyloidosis in individuals at risk of Alzheimer's disease (INSIGHT-preAD): a longitudinal observational study. Lancet Neurol. 2018;17:335–346. doi: 10.1016/S1474-4422(18)30029-2. [DOI] [PubMed] [Google Scholar]
- 3.Iwatsubo T., Iwata A., Suzuki K., Ihara R., Arai H., Ishii K. Japanese and North American Alzheimer's Disease Neuroimaging Initiative studies: Harmonization for international trials. Alzheimers Dement. 2018;14:1077–1087. doi: 10.1016/j.jalz.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Yamane T., Ishii K., Sakata M., Ikari Y., Nishio T., Ishii K. Inter-rater variability of visual interpretation and comparison with quantitative evaluation of 11C-PiB PET amyloid images of the Japanese Alzheimer's Disease Neuroimaging Initiative (J-ADNI) multicenter study. Eur J Nucl Med Mol Imaging. 2017;44:850–857. doi: 10.1007/s00259-016-3591-2. [DOI] [PubMed] [Google Scholar]
- 5.Landau S.M., Lu M., Joshi A.D., Pontecorvo M., Mintun M.A., Trojanowski J.Q. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol. 2013;74:826–836. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leuzy A., Chiotis K., Hasselbalch S.G., Rinne J.O., de Mendonça A., Otto M. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain. 2016;139:2540–2553. doi: 10.1093/brain/aww160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe C.C., Ellis K.A., Rimajova M., Bourgeat P., Pike K.E., Jones G. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 10.Mattsson N., Groot C., Jansen W.J., Landau S.M., Villemagne V.L., Engelborghs S. Prevalence of the apolipoprotein E ε4 allele in amyloid β positive subjects across the spectrum of Alzheimer's disease. Alzheimers Dement. 2018;14:913–924. doi: 10.1016/j.jalz.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Takei N., Miyashita A., Tsukie T., Arai H., Asada T., Imagawa M. Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese. Genomics. 2009;93:441–448. doi: 10.1016/j.ygeno.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Hassenstab J., Ruvolo D., Jasielec M., Xiong C., Grant E., Morris J.C. Absence of practice effects in preclinical Alzheimer's disease. Neuropsychology. 2015;29:940–948. doi: 10.1037/neu0000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mormino E.C., Papp K.V., Rentz D.M., Donohue M.C., Amariglio R., Quiroz Y.T. Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated amyloid β. Alzheimers Dement. 2017;13:1004–1012. doi: 10.1016/j.jalz.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papp K.V., Rentz D.M., Orlovsky I., Sperling R.A., Mormino E.C. Optimizing the preclinical Alzheimer's cognitive composite with semantic processing: The PACC5. Alzheimers Dement (N Y) 2017;3:668–677. doi: 10.1016/j.trci.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monsell S.E., Mock C., Hassenstab J., Roe C.M., Cairns N.J., Morris J.C. Neuropsychological changes in asymptomatic persons with Alzheimer disease neuropathology. Neurology. 2014;83:434–440. doi: 10.1212/WNL.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buerger K., Ewers M., Pirttilä T., Zinkowski R., Alafuzoff I., Teipel S.J. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 18.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]