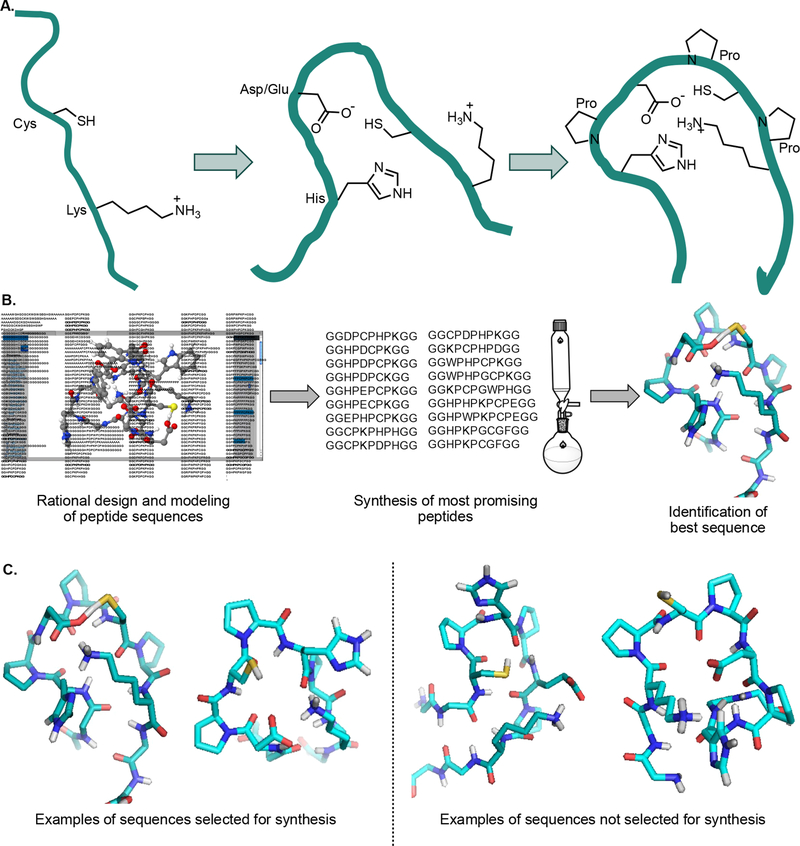
Figure 2.
Rational design of a peptide that covalently binds a CBT derivative. (A) Principles behind generating peptide sequences for computational modeling. Lysine and cysteine residues were interspersed with histidine and aspartic or glutamic acid residues to increase nucleophilicity. Prolines were added to lock the sequence (teal) into a favorable conformation for reaction with a CBT derivative. (B) Selection of sequences to test for reaction with a CBT derivative. 218 sequences were modeled using the program PEP-FOLD (see Supplemental Table S1 for a full list).26,27 Of these peptides, 18 that best fit our selection criteria were synthesized by solid-phase peptide synthesis (SPPS) and tested in a binding assay. (C) Examples demonstrating how peptides were selected for SPPS. The peptides on the left were two of the best models, whereas the two peptides on the right were not chosen for SPPS.