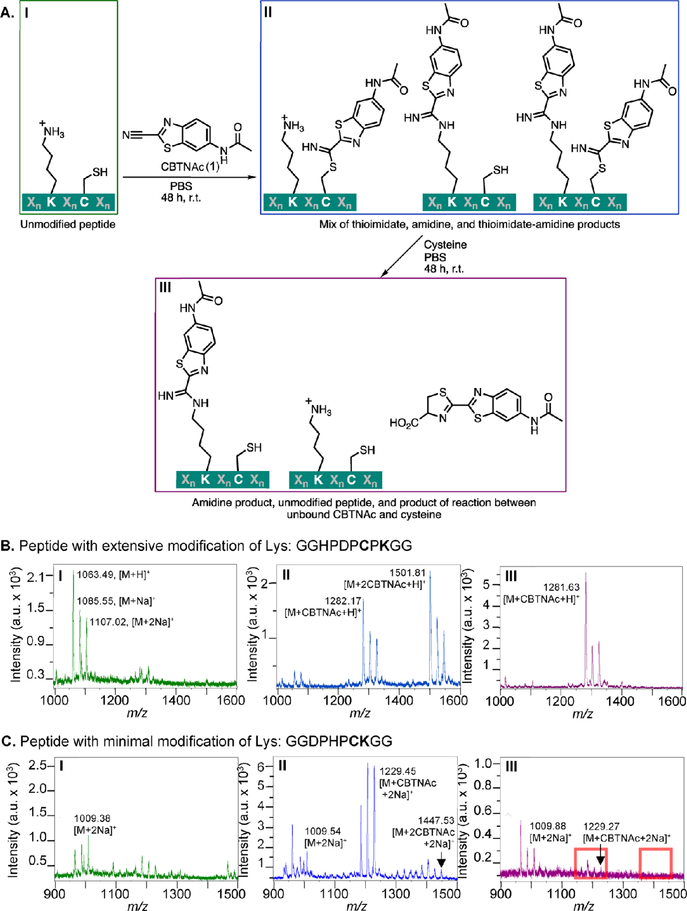
Figure 3.
Assay for peptides that are covalently modified by CBTNAc (1) and representative results. (A) Overview of assay used to determine degree of irreversible covalent modification of candidate peptides. Candidate N-terminal acetylated, C-terminal amidated peptides were incubated with CBTNAc in PBS for 48 h at room temperature, and then an aliquot of the reaction mixture was analyzed by MALDI-TOF MS. The remaining solution was incubated for 48 h at room temperature with free cysteine, with or without DTT or TCEP reducing agent, and the reaction mixture was again analyzed by MALDI-TOF MS. (B) Representative MALDI-TOF MS spectra at each stage of the assay for a peptide with a lysine residue extensively modified by CBTNAc (peptide 4). The first spectrum (I) shows crude peptide alone, the second spectrum (II) was taken after incubation with CBTNAc and desalting, and the third spectrum (III) followed incubation with free cysteine. (C) Representative MALDI-TOF MS spectra at each stage of the assay for a peptide with a lysine residue not significantly modified by CBTNAc (peptide 5). The samples analyzed in each spectrum were prepared the same way as in B. Red boxes indicate the expected locations of masses corresponding to modified peptide.