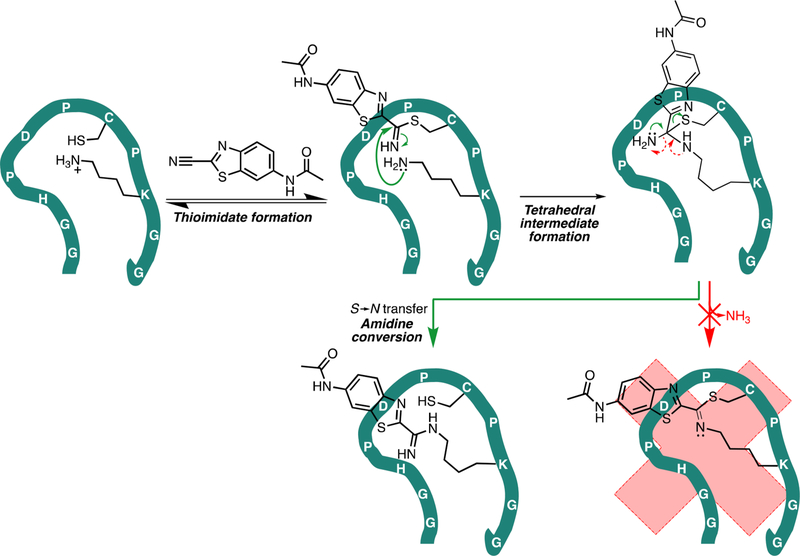
Figure 5.
Proposed mechanism of reaction between CBTag 1.0 and CBTNAc. First, the nucleophilic thiol on the cysteine residue reversibly forms a thioimidate bond with CBTNAc, likely catalyzed by the imidazole of the histidine residue.28,29 Then, the lysine amine attacks the thioimidate, resulting in a tetrahedral intermediate. Unlike the reaction to make firefly luciferin, a condensation step that releases ammonia does not proceed (red arrows). Instead, the end result is a stable amidine bond between the CBTNAc and the lysine residue in a “cysteine hand-off” mechanism analogous to that of NCL.