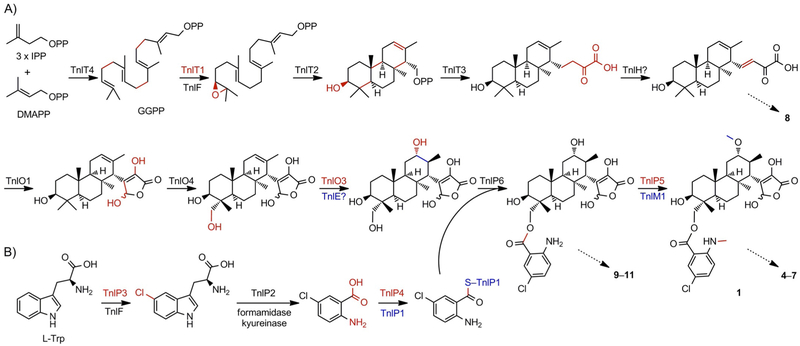
Scheme 3.
Proposed biosynthetic pathway for TNL A (1) supported by the isolation of congeners or shunt metabolites 4–11, as indicated by the dashed arrows. A) The four core proteins, TnlT1–T4, construct the diterpenoid scaffold and elongate the side chain. Then, a series of oxidative reactions form the γ-butyrolactone moiety and hydroxylate the C-18 and C-12 positions before acylation of the chloroanthranilate moiety. Finally, the N- and O-methylations, catalyzed by TnlP5 and TnlM1, respectively, complete the biosynthesis, although their timing is still unknown. IPP: isopentenyl diphosphate, DMAPP: dimethylallyl diphosphate, GGPP: geranylgeranyl diphosphate. B) Biosynthesis of the chloroanthranilate moiety from l-tryptophan follows that of tryptophan degradation through the kynurenine pathway. Chemical transformations are colored red; for multiple transformations in one step, red and blue are used.