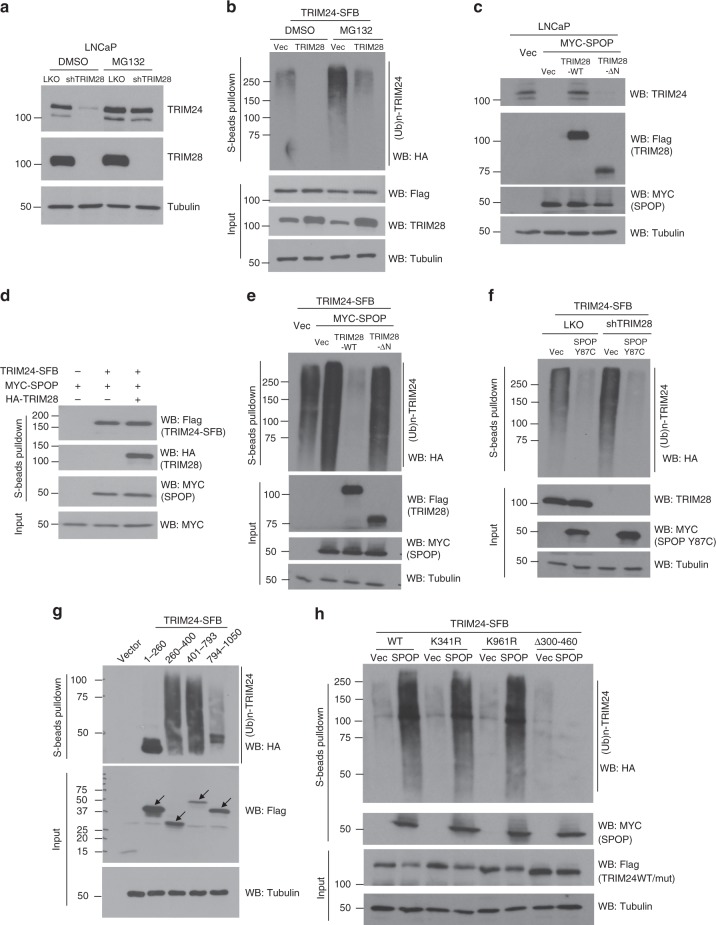
Fig. 3.
TRIM28 protects TRIM24 from SPOP-mediated ubiquitination and degradation. a Loss of TRIM28 causes proteasome-mediated degradation of TRIM24. LNCaP cells expressing shCtrl or shTRIM28 were treated with DMSO or 10 uM MG132 for 8 h and protein lysates were subjected to western blot analysis using indicated antibodies. b Overexpression of TRIM28 abolishes ubiquitination of TRIM24. HEK293T cells transiently overexpressing TRIM24-SFB, HA-Ub and empty vector or pCDNA-TRIM28 were treated with DMSO or 20uM MG132 for 4 h and then harvested. S-beads were used to pull-down TRIM24-SFB from cell extract and bound proteins were analyzed by immunoblotting using indicated antibodies. c TRIM28 abolishes SPOP-mediated degradation of TRIM24 protein. LNCaP cells with transient overexpression of either empty vector or Myc-SPOP with co-expression of vector, TRIM28-SFB or TRIM28-ΔN-SFB were harvested and subject to immunoblot analysis. d TRIM28 does not affect SPOP binding to TRIM24. HEK293T cells co-transfected with TRIM24-SFB and empty vector, Myc-SPOP or Myc-SPOP and HA-TRIM28 were treated with 20uM MG132 for 24 h and then harvested. S-beads were used to pull-down TRIM24-SFB from cell extract, bound proteins were analyzed by immunoblotting using indicated antibodies. e TRIM28 overexpression diminishes SPOP-mediated ubiquitination of TRIM24. S-beads pull-down was performed using protein extracts of MG132-treated HEK293T cells co-expressing TRIM24-SFB and HA-Ub and empty vector, MYC-SPOP, MYC-SPOP and Flag-TRIM28 or MYC-SPOP and Flag-TRIM28-ΔN. f TRIM28 knockdown enables SPOP-mediated ubiquitination of TRIM24. LNCaP cells were transfected with TRIM24-SFB, HA-Ub, and various constructs as indicated. After 4 h of 20 uM MG132 treatment, cells were harvested and cell lysates were subjected to S-beads pull-down followed by immunoblotting. g Various TRIM24-SFB deletion constructs were co-transfected with HA-Ub. Cells were treated with 20 µM MG132 treatment for 4 h and S-beads was used to pull-down TRIM24-SFB fragments. Ubiquitinated TRIM24 was detected by anti-HA antibody. h Ubiquitination of TRIM24 by SPOP occurs between 300–460aa. Wild type, K341R, K961R, and Δ300–460 mutants of TRIM24-SFB were co-transfected with HA-Ub with or without Myc-SPOP. Cells were treated with 20 µM MG132 treatment for 24 h and S-beads was used to pull-down TRIM24-SFB wild type or mutants. Ubiquitinated TRIM24 was detected by anti-HA antibody