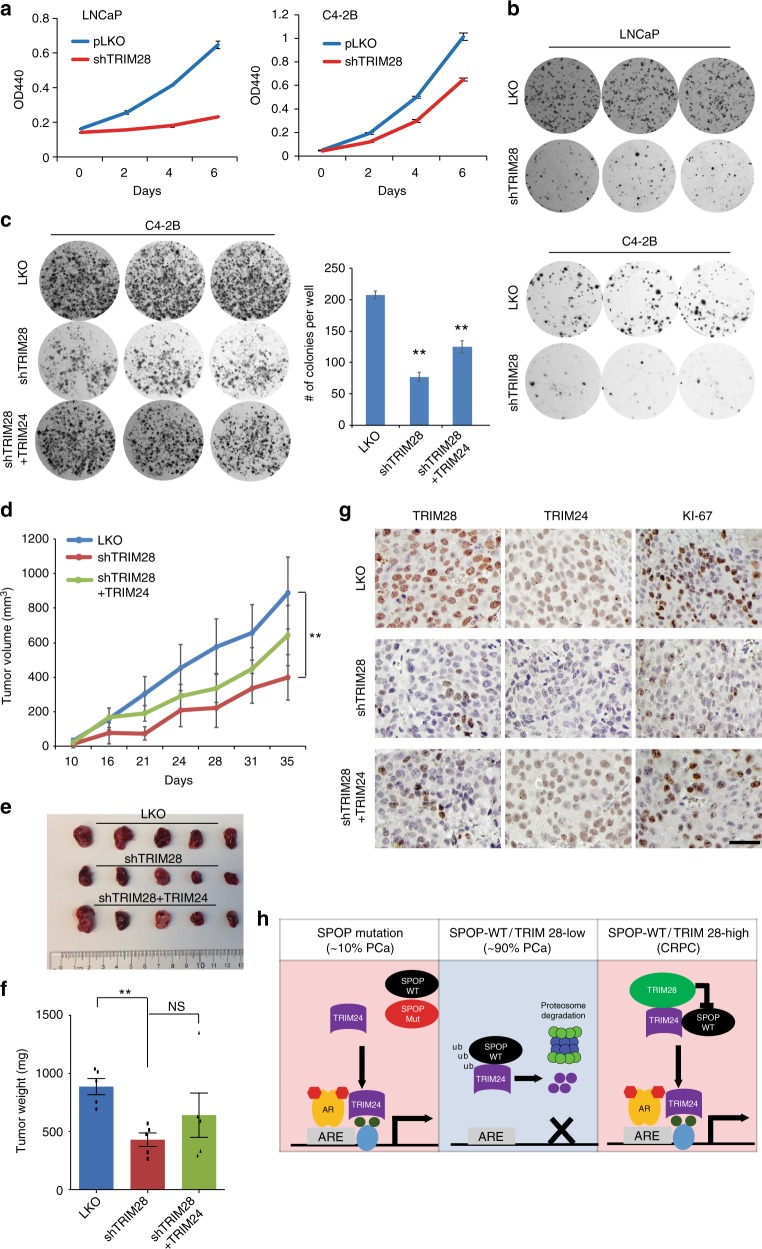
Fig. 7.
TRIM28 promotes prostate cancer tumorigenesis in vitro and in vivo. a, b. TRIM28 is required for prostate cancer growth. Proliferation of control or TRIM28-knockdown LNCaP and C4-2B cells with were evaluated by WST-1 assay (a) and colony formation assay (b). c Colony formation assay was performed using C4-2B cells transduced with lentiviral supernatant of pLKO, shTRIM28, and shTRIM28 along with TRIM24-SFB. After 10–14 days, colonies were stained, imaged, and quantified in a bar graph. Data shown is mean (± SEM, n = 3). **P < 0.01 by Student’s t-test. d–g TRIM28 depletion reduced CRPC xenograft tumor growth in part through decreasing TRIM24. C4-2B cells (4 × 106) expressing pLKO, shTRIM28, or shTRIM28 along with TRIM24-SFB were inoculated to the right flanks of CB17-SCID mice. Tumor growth was measured twice per week (d) and the difference among groups were determined by ANOVA (**p < 0.001). Tumors were excised at the endpoint (e) and weighed (f). Statistical differences of endpoint tumor weight between experimental and control group was determined by Student’s t-test. Data shown is mean (± SEM, n = 5). *P < 0.05; NS: not significant. Tumor tissues from mice were paraffin embedded, sectioned and subjected to immunohistochemistry with the use of anti-TRIM28, TRIM24 and KI-67 antibodies as indicated (g). Scale bar = 50 µm. h A model depicting broad TRIM24 upregulation by TRIM28 in CRPC. In PCa with SPOP mutations (~ 10% PCa, 7% CRPC), mutant SPOP forms heterodimers with wild-type SPOP to prevent it from binding to and degradation of TRIM24, which subsequently leads to TRIM24 protein increase and enhanced AR transcriptional activities. In a majority of localized PCa that are SPOP wild type and TRIM28 low, SPOP recruits Culin3-RBX1 ubiquitin ligase to TRIM24 for ubiquitination and subsequent degradation in proteasomes. In CRPC wherein TRIM28 is highly elevated, TRIM28 binds to TRIM24 protein to prevent its ubiquitination by SPOP and stabilize TRIM24, thereby enhancing AR signaling and driving CRPC tumor growth